Abstract
Aim
Raised intraocular pressure (IOP) increases the risk of glaucoma. Eye-care professionals measure IOP to screen for ocular hypertension (OHT) (IOP>21 mm Hg) and to monitor glaucoma treatment. Tonometers commonly develop significant systematic measurement errors within months of calibration, and may not be verified often enough. There is no published evidence indicating how accurate tonometers should be. We analysed IOP measurements from a population study to estimate the sensitivity of detection of OHT to systematic errors in IOP measurements.
Methods
We analysed IOP data from 3654 participants in the Blue Mountains Eye Study, Australia. An inverse cumulative distribution indicating the proportion of individuals with highest IOP>21 mm Hg was calculated. A second-order polynomial was fitted to the distribution and used to calculate over- and under-detection of OHT that would be caused by systematic measurement errors between −4 and +4 mm Hg. We calculated changes in the apparent prevalence of OHT caused by systematic errors in IOP.
Results
A tonometer that consistently under- or over-reads by 1 mm Hg will miss 34% of individuals with OHT, or yield 58% more positive screening tests, respectively. Tonometers with systematic errors of −4 and +4 mm Hg would miss 76% of individuals with OHT and would over-detect OHT by a factor of seven. Over- and under-detection of OHT are not strongly affected by cutoff IOP.
Conclusion
We conclude that tonometers should be maintained and verified at intervals short enough to control systematic errors in IOP measurements to substantially less than 1 mm Hg.
Similar content being viewed by others
Introduction
Intraocular pressure (IOP) greater than 21 mm Hg, commonly classified as ocular hypertension (OHT),1 substantially increases the risk of glaucoma.2 Glaucoma, a neurodegenerative disease of the retinal ganglion cells, is the second leading cause of blindness worldwide.3 In the United States, 4 million people have glaucoma and 130 000 are legally blind from the disease.3 In the Australian Blue Mountains Eye study of adults 49 years of age and older, the prevalence of open-angle glaucoma was 3% and OHT was found in 3.7% of participants.1 Control of IOP is important in patients with glaucoma:4 a decrease of 1 mm Hg may lead to a 10% reduction in the risk of progressive nerve damage.5, 6 Reduction and control of IOP are the only effective treatments for glaucoma.
IOP is measured by eye-care professionals to screen for, diagnose, treat and monitor OHT and eye diseases such as glaucoma. The Goldmann applanation tonometer in experienced hands is recognised as the gold standard for IOP measurements.7 Both Goldmann applanation and non-contact tonometers are known to produce clinically significant systematic errors if inadequately maintained and calibrated,8, 9, 10, 11, 12, 13, 14, 15 and current calibration intervals may be too long.8, 9, 12, 14, 16, 17, 18
Systematic errors in IOP measurements are likely to lead to over- or under-detection of OHT. Over-detection of OHT during screening may result in excessive referrals to ophthalmologists, while under-detection may place individuals at increased risk of glaucoma. Inadequate calibration or variability of calibration19 of tonometers used for treating or monitoring patients with OHT or glaucoma may cause apparently random variations in IOP, even if monitoring is performed on a single tonometer.
The effects of systematic errors in IOP measurements on over- and under-detection of OHT and treatment of glaucoma are not known and have not been studied. There is no published quantitative evidence indicating how accurate tonometers should be. In this study, we analysed IOP measurements from a population study to estimate the sensitivity of detection of OHT to systematic errors in IOP measurements.
Materials and methods
We analysed valid IOP measurements obtained in the Blue Mountain Eye Study (BMES).1 The BMES was approved by the Western Sydney Area Health Service Human Ethics Committee, and written, informed consent was obtained from all participants. All IOP measurements were performed using a single Goldmann applanation tonometer and slit lamp (both Haag-Streit, Bern, Switzerland) after a drop of Fluress (benoxinate hydrochloride 0.4%, sodium fluorescein 0.25%, Barnes-Hind, Sunnyvale, CA, USA). A single measurement was taken and repeated if judged unreliable. All IOP measurements were performed by the same operator (a trained study orthoptist), during the 3-year course of the BMES. Any IOP measurements less than 10 or greater than 21 mm Hg were repeated by the Principal Investigator in the BMES (PM). The tonometer was calibrated regularly (at least 3 times per year during the course of the study).
We analysed all valid left, right and highest IOP measurements from all participants using a method similar to that published earlier for analysing blood pressure distributions.20 IOP measurements were assumed to be rounded to the nearest mm Hg: histograms and cumulative distributions were calculated using thresholds at midpoints between integer IOP values. An inverse cumulative distribution indicating the percentage of subjects with IOPs greater than the abscissa value was calculated by summing the histograms and subtracting the sums from 100. First-, second- and third-order polynomials were fitted to the logarithm of the cumulative distribution by linear regression to smooth the distribution and facilitate interpolation. Goodness of fit of the polynomials was assessed by examining R2, the standard errors of the regressions and the statistical significance of the coefficients. Confidence intervals (95%) around mean predicted values were calculated.21 The proportion of subjects who had OHT (IOP>21 mm Hg) was estimated from the polynomial. To estimate the effects of systematic measurement errors, the proportion was re-estimated assuming the tonometer systematically over- and under-read by 0–4 mm Hg at 0.1 mm Hg intervals. The sensitivity of over- and under-detection of OHT to the cutoff IOP was investigated by repeating the analysis for cutoff IOPs of 20 and 22 mm Hg. Data analysis was performed using Matlab 2009b (Mathworks, Nattick, MA, USA).
Results
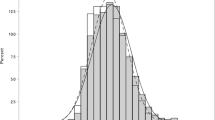
Histograms of right and left IOP measurements are shown in Figure 1. The difference between the IOP of left and right eyes was 0.09 ±1.95 (mean±SD) mm Hg and the Pearson correlation coefficient was 0.78. Results for left, right and highest IOPs were similar, therefore we report results for highest IOPs only. The cumulative distribution indicating the percentage of subjects with highest IOP greater than 17.5–27.5 mm Hg is shown in Figure 2, in which the vertical scale is logarithmic. The SE of the first-, second- and third-order regressions were 0.0900, 0.0219, and 0.0233 logarithmic units, corresponding to 1.230, 1.052, and 1.055 percentage points, respectively. Respective R2 values were 0.975, 0.999, and 0.999. The coefficients of the second-order polynomial were all significantly different from 0 at the 95% level of confidence. Therefore, the relationship between the logarithm of the inverse cumulative distribution and IOP is described best by a second-order polynomial.
Histograms of all baseline intraocular pressures measured in the Blue Mountains Eye Study.1
Inverse cumulative distributions showing proportions (%) of participants with highest IOP greater than the abscissa value. Lines above and below the regression line indicate 95% confidence interval (CI) of predicted mean value. Vertical and horizontal lines indicate percentages of participants (with corresponding 95% CI) in whom ocular hypertension (IOP>21 mm Hg) would be detected when the tonometer has a systematic error of −2, 0, and +2 mm Hg, respectively.
Over- and under-detection of OHT (highest IOP>21 mm Hg) caused by systematic errors of ±2 mm Hg are indicated by the construction lines in Figure 2. Changes in the apparent prevalence of OHT that would be caused by systematic errors in IOP measurements of ±1, ±2, ±3 and ±4 mm Hg are shown in Table 1. Figure 3a and b show the effects of systematic errors in IOP on the detection of OHT over the continuous ranges 0 to +4 and −4 to 0 mm Hg, respectively, using cutoff IOPs of 20, 21, and 22 mm Hg.
Over-detection of ocular hypertension caused by positive systematic measurement errors (a), and proportion of individuals with ocular hypertension who would be missed due to negative systematic measurement error (b). The dotted and broken lines indicate the effects of variations in cutoff IOP on over- and under-detection.
Discussion
In this study, we show that detection of OHT is very sensitive to systematic errors in IOP measurements. When a population is screened for OHT, a tonometer that over-reads by 1 mm Hg may result in the number of positive results increasing by ∼58% (Figure 3a) or ∼3.3% of the population (Table 1). Conversely, a tonometer that under-reads by 1 mm Hg may result in ∼34% of individuals with OHT (1.9% of the population) being missed (Figure 3b). Systematic errors of +1.5 mm Hg and −1.7 mm Hg may result in the number of individuals with detected OHT doubling or halving, respectively. Larger systematic errors of ±4 mm Hg may result in the number of positive screening results increasing by a factor of seven (Figure 3a), or 76% of individuals with OHT being missed (Figure 3b). Some patients whose IOP is screened with an inadequately calibrated tonometer may be referred to an ophthalmologist unnecessarily and, some patients with OHT may not be referred and be at unnecessarily increased risk of glaucoma.
Figure 3a and b shows that under- and over-detection of OHT does not depend strongly on the IOP cutoff used to define OHT. For example, at a systematic error of +3 mm Hg, a change of cutoff from 21–22 mm Hg changes over-detection from 345 to 293 (Figure 3a), a change of only (293−345)/345×100=−15%. An earlier study that showed when the logarithm of the inverse cumulative distribution is linear, variations in the cutoff have no effect on over- and under-detection.20 The weak non-linearity shown in Figure 2 is associated with small changes in under- and over-detection of OHT with changes in IOP cutoff.
Systematic error or bias may be common in both non-contact and applanation tonometers in regular clinical use. Three non-contact tonometers evaluated against a calibrated Goldmann applanation tonometer exhibited mean errors of 0.5, 2.9, and 1.8 mm Hg after 20, 9, and 24 months of operation, respectively.8 Choudhari et al9 checked the calibration of 132 Goldmann applanation tonometers, using the standard weight bar supplied by the manufacturer, 6 months after they had been calibrated by hospital biomedical engineers, and found calibration errors ranging from −8 to +20 mm Hg. Intra- and inter-observer limits of agreement for duplicate calibrations were up to 4 mm Hg.9 At 20 mm Hg, 55%, 10%, and 5% of tonometers had errors greater than or equal to 2, 4, and 6 mm Hg respectively.9 Only 4% of tonometers complied with the manufacturer’s tolerance of ±0.5 mm Hg. Sandhu et al12 found that 6 months after 34 Goldmann tonometers had been calibrated by a specially designated technician, 41% exhibited calibration errors of 3 mm Hg or greater at a nominal 20 mm Hg. Four months later, 52% exhibited calibration errors of 3 mm Hg or greater.12 George et al22 assessed in vivo agreement between two calibrated Goldmann applanation tonometers from different manufacturers and found limits of agreement of −2.47 to +6.16 mm Hg. Systematic measurement errors may also be caused by distortions of the applanating surface of disposable tonometer heads.23
In a survey of 155 ophthalmology units in the United Kingdom, Kumar et al17 found that 39% of units checked the calibration of their Goldmann tonometers either never or in a random manner. In a separate UK survey of ophthalmology residents, 85% never checked tonometers and 70% felt that calibration checks were not part of their responsibility.16 Only 7% checked the tonometer at the start of each clinical session and 8% would only check the tonometer if they had suspicious or unexpected measurements.16
IOP is not the only variable used by ophthalmologists to estimate risk of loss of vision. Glaucoma occurs in many patients whose IOP is below 21 mm Hg. Clinical optic disc examination and other quantitative measurements24 contribute to the detection, treatment and management of glaucoma. IOP, however, is an important risk factor for glaucoma25 measured to screen for OHT, and it is important that the measurement is reliable.
Intra-individual variation of IOP can be several mm Hg,26, 27 often substantially larger than calibration errors, possibly leading to an assumption that small systematic errors are not important. Systematic errors are approximately constant and create a bias at every IOP reading in every individual. The effect is similar to a shift in the population distribution of IOP. Decisions regarding treatment or follow-up are made, explicitly or implicitly, for every patient examined. On average, over a large number of patients, a small positive systematic error will result in more patients being treated or referred for further investigation, and a small negative systematic error will result in some patients not being treated or referred, who otherwise would be. This study quantifies those effects and shows that the influence of small systematic errors may be surprisingly large.
Important limitations of this study include the intra-individual variability of IOP of the participants and the possibility of undetected calibration errors in the tonometers used in the Blue Mountains Eye Study. Intra-individual variability and random calibration errors would increase the inter-individual IOP variance, broaden the IOP distribution and hence reduce the slope of the inverse cumulative distribution of IOP. This slope reduction would reduce the apparent sensitivity of OHT detection to tonometer calibration errors. Hence, this study may under-estimate the sensitivity.
We conclude that tonometers should be maintained and properly calibrated at intervals short enough to control systematic errors in IOP measurements to substantially less than 1 mm Hg.

References
Mitchell P, Smith W, Attebo K, Healey PR . Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology 1996; 103 (10): 1661–1669.
Kwon YH, Fingert JH, Kuehn MH, Alward WL . Primary open-angle glaucoma. N Engl J Med 2009; 360 (11): 1113–1124.
Noecker RJ . The management of glaucoma and intraocular hypertension: current approaches and recent advances. Ther Clin Risk Manag 2006; 2 (2): 193–206.
Chihara E . Assessment of true intraocular pressure: the gap between theory and practical data. Surv Ophthalmol 2008; 53 (3): 203–218.
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M . Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002; 120 (10): 1268–1279.
Bengtsson B, Leske MC, Hyman L, Heijl A . Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology 2007; 114 (2): 205–209.
Sheehan W, Adams D, Wells C, Booth A . Does Goldmann applanation tonometry performed by community optometrists reduce referrals? A pilot study. Br J Ophthalmol 2011; 95 (2): 295.
Atkinson PL, Wishart PK, James JN, Vernon SA, Reid F . Deterioration in the accuracy of the Pulsair non-contact tonometer with use: need for regular calibration. Eye (Lond) 1992; 6 (Pt 5): 530–534.
Choudhari NS, George R, Baskaran M, Vijaya L, Dudeja N . Measurement of Goldmann applanation tonometer calibration error. Ophthalmology 2009; 116 (1): 3–8.
Kumar N, Jivan S, Batterbury M . Tonometer calibration. Ophthalmology 2009; 116 (12): 2480–2481.
Chuo JY, Mikelberg FS . Calibration errors of Goldmann tonometers in a tertiary eye care centre. Can J Ophthalmol 2007; 42 (5): 712–714.
Sandhu SS, Chattopadhyay S, Birch MK, Ray-Chaudhuri N . Frequency of goldmann applanation tonometer calibration error checks. J Glaucoma 2005; 14 (3): 215–218.
Wessels IF, Oh Y . Tonometer utilization, accuracy, and calibration under field conditions. Arch Ophthalmol 1990; 108 (12): 1709–1712.
Tattersall C, Vernon S, Wood C . Improving the accuracy of intraocular pressure readings. Nurs Times 2006; 102 (27): 36–38.
Brandt JD . The myth of clinical precision. Ophthalmology 2009; 116 (1): 1–2 e1.
Kumar N, Jivan S . Goldmann applanation tonometer calibration error checks: current practice in the UK. Eye (Lond) 2007; 21 (6): 733–734.
Kumar N, Hillier RJ . Goldmann tonometer calibration: a national survey. Eye (Lond) 2009; 23 (2): 413–415.
Ng J, Kashani S, Qureshi K, Ferguson V . Intraocular pressure measurement and goldmann calibration. An eye opening review of practice in United Kingdom. Eye (Lond) 2007; 21 (6): 851–853.
Choudhari NS, Jadhav V, George R, Vijaya L . Variability in the calibration error of the goldmann applanation tonometer. J Glaucoma 2011; 20 (8): 492–496.
Turner MJ, Baker AB, Kam PC . Effects of systematic errors in blood pressure measurements on the diagnosis of hypertension. Blood Press Monit 2004; 9 (5): 249–253.
Draper NR, Smith H . Applied Regression Analysis 3rd ed. Wiley-Interscience: New York, 1998.
George R, Arvind H, Baskaran M, Ramesh SV, Raju P, Vijaya L . Agreement between two Goldmann type applanation tonometers. Indian J Ophthalmol 2008; 56 (6): 516–517.
Goel S, Chua C, Dong B, Butcher M, Ahfat F, Hindi SK et al. Comparison between standard Goldmann applanation prism and disposable applanation prism in tonometry. Eye (Lond) 2004; 18 (2): 175–178.
Quigley HA . Glaucoma. Lancet 2011; 377 (9774): 1367–1377.
Medeiros FA, Weinreb RN . Estimating the risk of developing glaucoma. Open Ophthalmol J 2009; 3: 50–53.
Wilensky JT . Diurnal variations in intraocular pressure. Trans Am Ophthalmol Soc 1991; 89: 757–790.
Wilensky JT, Gieser DK, Dietsche ML, Mori MT, Zeimer R . Individual variability in the diurnal intraocular pressure curve. Ophthalmology 1993; 100 (6): 940–944.
Acknowledgements
We gratefully acknowledge the assistance of George Burlutsky and Elena Rochtchina at Clinical Ophthalmology and Eye Health, Westmead Clinical School, C24 —Westmead Hospital, The University of Sydney. MJT was supported by an Innovation Fellowship from Macquarie University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Turner, M., Graham, S., Avolio, A. et al. Potential effects of systematic errors in intraocular pressure measurements on screening for ocular hypertension. Eye 27, 502–506 (2013). https://doi.org/10.1038/eye.2013.7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2013.7
Keywords
This article is cited by
-
Novel dynamic corneal response parameters in a practice use: a critical review
BioMedical Engineering OnLine (2019)