Abstract
Background
To evaluate the optimal compression level of retinal color digital video recordings, a novel video-based imaging technology, in screening for diabetic retinopathy (DR).
Design
Evaluation of a diagnostic technique.
Methods
A total of 36 retinal videos, captured using EyeScan (Ophthalmic Imaging System), were compressed from original uncompressed file size of 1 GB (gigabyte) to four different compression levels—100 MB (megabyte) (Group 1); 30 MB (Group 2); 20 MB (Group 3); and 5 MB (Group 4). The videos were subsequently interpreted by an ophthalmologist and a resident using the International Clinical Diabetic Retinopathy Severity Scales.
Main outcome measures
The sensitivity, specificity and κ coefficient for DR grading detected by were calculated for each compression level (Groups 1–4), with reference to the original uncompressed retinal videos.
Results
Groups 1, 2, and 3 graded by both readers had sensitivity and specificity >90% in detecting DR, whereas for group 4, the sensitivity and specificity were 70.6% and 94.7% for ophthalmologist and 80.0% and 72.2% medical officer, respectively. The κ correlation in detecting DR for groups 1, 2, and 3 were >0.95, whereas for Group 4, the κ was 0.76 and 0.66 for ophthalmologist and medical officer, respectively.
Conclusion
Retinal video recording is a novel and effective DR screening technique with high sensitivity, specificity and κ correlation. With its compressibility, this is a potential effective technique that can be widely implemented in a routine, mobile, and tele-ophthalmology setting for DR screening services.
Similar content being viewed by others
Introduction
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia secondary to either impaired insulin secretion or insulin resistance. The chronic uncontrolled hyperglycemia can give rise to macrovascular (cerebrovascular accident, ischemic heart disease, and peripheral vascular disease) and microvascular (retinopathy, nephropathy, and neuropathy) complications. Diabetic retinopathy (DR) is one of the commonest microvascular complications of diabetes. Nearly 100% type 1 diabetic patients and >60% type 2 diabetic patients will have at least some retinopathy after 20 years of diabetes.1, 2 Therefore, it is crucial for primary eye care providers to regularly screen people with diabetes for DR, as early detection can prevent severe visual impairment.3
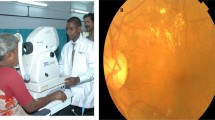
Traditionally, retinal still photography has been the gold standard DR screening tool in the primary health-care setting. However, the video-based imaging technology using retinal video recording has been recently proposed to be a novel DR screening method.4 This technique is quick to perform, easy to learn (single-day training) and does not require any previous ophthalmic imaging experience. Compared with the retinal still photography, the retinal video not only provides a greater field within shorter period of time but also mimics what is seen with a slit lamp examination. Nevertheless, this technique was limited by its large video file size, which requires high storage capacity.4 On average, a retinal video of 30 s takes up ∼500 MB and thus, will be impractical to archive and transmit such large video files during routine DR screenings.
The objective of our study is to investigate and determine the optimal compression level for retinal videos to screen for DR. The effect of retinal still image compression for DR screening has been studied previously5, 6, 7, but none of the which has investigated the use of retinal videos compression technique. Compression of retinal videos will help to reduce the need for a large storage capacity and to increase the data transmission speed for DR screening in a routine, mobile and tele-ophthalmology setting.
Materials and methods
Sample population
A total of 36 retinal videos (18 normal and 18 with DR changes) at four different compression levels—100 MB (Group 1), 30 MB (Group 2), 20 MB (Group 3), and 5 MB (Group 4), captured by retinal video recording using OIS EyeScan (Ophthalmic Imaging System, Sacramento, CA, USA)—were selected for our study. Given that three-field (optic disc, macula, and temporal views) retinal still photogaphy has been the standard of care for patients with diabetes in our center, the retinal video recordings were also captured in a similar manner for the recruited patients in our study. All study subjects were enrolled from the DR Screening Clinic of Royal Perth Hospital and this study has been approved by the Royal Perth Hospital Human Research Ethics Committee.
Sample size estimation
To allow for a power of 95%, desired precision of 0.10, expected sensitivity and specificity of 96%, the total number of eyes required for each compression level was 31 (prevalence was set at 0.50 as selected samples consisted of 50% normal and 50% abnormal retinal digital videos).
Conversion process
A digital video can exist in various different file formats such as moving picture experts group (MPEG), audio video interleave (AVI), windows media video (WMV), QuickTime Movie File (MOVIE), and, so on. It consists of a series of bitmap digital images displayed in a rapid succession at a constant rate. The size of a video is determined by bit rates (BRs) and time (T). BR, defined as the number of bits processed or conveyed per unit time, represents the amount of information stored per unit of time of a recording. It is determined by the frame rate (FR), frame size (FS) and color depth (CD) of a video. The FR is defined as the number of displayed digital images per unit time and it is often measured in a second (frames per second; FPS). The FS is the total number of pixels in terms of width (W) and height (H) of an image. The CD represents the amount of bits that form a single pixel. By altering one of these properties, one can modify the size of a digital video using various video compression software packages and codecs, which are readily available on the internet.
For our study, the uncompressed raw retinal videos were compressed to four different levels (Groups 1–3, 4) from the original file size using a video converter software, Xilisoft Video Converter Ultimate 6.0 (Xilisoft Corporation, British Virgin Island), which utilizes a standard video codec H.264 (Table 1). The average size of an original uncompressed 30-s video was 500 MB. In our study, we reduced the 500-MB video file to four different levels (Group 1: 100 MB, Group 2: 30 MB, Group 3: 20 MB, and Group 4: 5 MB) by changing the BRs. The FR and FS were set at 17 FPS and 640 × 480 pixels, respectively, as these settings have been preset by the fundus camera (OIS EyeScan).
Reference standard
We utilized the raw uncompressed raw retinal videos as the reference of our study as from our previous study,4 the sensitivity and specificity of an uncompressed raw retinal videos in detecting any grade of DR by a retinal specialist and a consultant ophthalmologist with special interest in diabetes, compared with the slit lamp examination by a senior consultant ophthalmologist (with >10 years experience in screening DR), were >90%.
Data interpretation
The converted videos were randomized and sent to two different readers (a consultant ophthalmologist and a medical officer) who were blinded to the compression level of the videos. Each reader interpreted a total of 144 retinal videos (36 retinal videos at four different compression levels) using a standard monitor screen (iMac 27’, Apple, Cupertino, CA, USA) with a VLC media player 1.1.4 (Apple) in a dimly lit room. The International Clinical DR Severity Scales8 was utilized to interpret and grade the DR severity by determining the presence/absence of lesions including microaneuryms, retinal hemorrhages, cotton wool spots, venous beading, intraretinal microvascular abnormalities, new vessels formation, vitreous hemorrhage, preretinal hemorrhage, and hard exudates. In addition, the retinal videos were rated as ‘acceptable’, ‘pixelated but interpretable’ and ‘unacceptable’ by the readers.
Data analysis
The data analysis was performed using SPSS version 17 (SPSS, Chicago, IL, USA). The main outcome measures were the sensitivity, specificity, and κ coefficient between the uncompressed raw retinal videos and compressed retinal videos in diagnosing any level of DR. In addition, the κ correlation was performed for other variables, such as (1) the microaneurysms and retinal hemorrhages between the uncompressed and compressed retinal videos and; (2) the DR grading between the consultant ophthalmologist and medical officer using the uncompressed retinal videos. Values of κ of 0.8 and above were considered as excellent agreement between two groups for our study.9
Results
All retinal videos in Group 1 (100 MB), 2 (30 MB), and 3 (20 MB) were rated as ‘acceptable’ by the ophthalmologist and medical officer (Table 2). For Group 4 (10 MB), only 11% and 3% of the retinal videos were rated as ‘acceptable’ by the ophthalmologist and medical officer, respectively. Of the 18 retinal videos with DR, 22% (n=4) had mild non-proliferative DR (NPDR), 61% (n=11) had moderate NPDR, 11% (n=2) had severe NPDR, and 6% (n=1) had proliferative DR.
The conversion time for the retinal videos to different compression levels are shown in Table 3. Using the uncompressed videos as gold standard, Group 1, 2, and 3 graded by both readers had excellent sensitivity and specificity of >90% in detecting DR (Table 4). On the other hand, Group 4 had the lowest sensitivity and specificity in detecting DR changes (ophthalmologist 1—sensitivity: 70.6%, specificity: 94.7%; medical officer: sensitivity: 80.0%; and specificity: 72.2%).
Also, the κ between the gold standard uncompressed videos and Groups 1, 2, and 3 to detect DR-related changes for both readers were >0.96, whereas for Group 4, the κ for the ophthalmologist and medical officer were 0.76 and 0.66, respectively. On the other hand, the κ between the ophthalmologist and medical officer for raw uncompressed videos, Group 1, Group 2, Group 3, and Group 4 were 0.84, 0.84, 0.80, 0.76, and 0.43, respectively. Similarly, the κ correlation of the microaneurysms, retinal hemorrhages, detected by both readers for Groups 1, 2, and 3 with reference to the gold standard video files (1 GB) were >0.9 (Table 5). For Group 4, the κ for the detection of microaneurysms by the ophthalmologist and medical officer were both 0.87, whereas for retinal hemorrhages, they were 0.88 and 0.87, respectively. The κ correlation of new vessel formation and subhyaloid hemorrhage was 1.0 in groups 1–4 graded by both ophthalmologist and medical officer, with reference to the uncompressed videos.
Discussion
Our study showed that the retinal videos could be significantly compressed down to 20 MB of its original file size with excellent sensitivity (ophthalmologist: 94.4%; medical officer: 100%) and specificity (ophthalmologist: 100%; medical officer: 93.8%) in detecting DR changes. The file size of a compressed 30-s retinal video consisting of optic disc, macula, and temporal views is ∼20 MB. For an uncompressed color fundus photo captured by FF450 plus (Carl Zeiss Meditec, Inc., Dublin, CA, USA) in the tagged image file format (TIFF) or BIT MAP format, each photo takes up ∼13 MB. In other words, a total of 39 MB is required for a three-field color retinal still photography. In comparison, a compressed video will have a lower storage capacity compared with the three uncompressed raw color fundus photos.
For Group 4 (5 MB), most of the retinal videos were rated as ‘pixelated but interpretable’ by both readers (Table 2). Nonetheless, they did not possess comparable sensitivity and specificity with other three groups (Table 4). In addition, the feedback from the ophthalmologist and medical officer were similar. Although reading the video recordings from groups 1–3 was comfortable, interpreting the ‘pixelated’ videos was ‘extremely tiring’ and ‘time consuming’, as more time was required to differentiate a true lesion from the normal retina and most of them were very ‘disjointed’ and ‘blurred’. Hence, this compression level will not be ideal in the setting of diabetic retinopathy screening.
To the authors’ knowledge, no data were published on retinal digital video recording for DR screening. Given that this is one of the first studies that evaluate the effectiveness of retinal video recordings at different compression levels, we selected the uncompressed raw retinal videos, which are considered to be of ‘good’ quality. Our results indicated that a retinal video can be compressed down to 20 MB without compromising its quality and diagnostic accuracy. This study will need to be further expanded on retinal videos with varying quality, especially ones with compromised quality due to media opacities and dark fundi, to assess the effects of video compression.
The medical officer in this study had interpreted >1000 color fundus photos of diabetic patients before this study. The difference in the detection of DR between the ophthalmologist and medical officer was minimal (Table 4). These results indicated that the compressed retinal videos could be potentially interpreted by trained non-ophthalmologist personnel and thus, the consultant specialist input can be redirected to more useful areas such as provision of consultations and surgical intervention for patients with sight-threatening DR.
Only one retinal video was graded differently to the uncompressed recording by the consultant ophthalmologist in Groups 2 and 3 and medical officer in Group 3. Owing to the relatively small sample size, this has significantly reduced the sensitivity and specificity of detecting DR grading by the consultant ophthalmologist (specificity of 94.6% in Group 2 and sensitivity of 94.4% in Group 3) and medical officer (specificity of 93.8% in Group 3) (Table 4). Despite fulfilling the sample size estimation, we felt that this was still one of the weaknesses of our study and thus, further research with a larger sample size will be of great value to explore the diagnostic accuracy of video compression at 30 and 20 MB, and other compression levels such as 15 and 10 MB.
In detecting DR changes for Groups 3 (20 MB) and 4 (5 MB), the sensitivity of the medical officer in detecting DR changes was higher than the ophthalmologist, but in contrast, the ophthalmologist had a higher specificity than the medical officer (Table 4). The difference in diagnostic sensitivity and specificity indicated that the medical officer had a lower threshold in diagnosing a patient with DR compared with the ophthalmologist, especially in cases where he was uncertain about a suspicious lesion. Although false-positive diagnoses may result in more ‘unnecessary’ referrals to an ophthalmologist, it is critical to note that a screener should always refer the patients with a suspicious lesion to an ophthalmologist to avoid any misdiagnosis of a sight-threatening condition.
The Xilisoft Video Converter Ultimate 6.0 utilizes a standard video codec H.264 which is compatible for both Windows and Macintosh. The retinal video recording device (OIS EyeScan) does not possess a built-in retinal video compression program, which performs different compression levels for the retinal videos. Given that the OIS EyeScan is one of the first fundus cameras to perform color retinal video recording, future research could be conducted for OIS EyeScan or other devices to incorporate a built-in retinal video compression program to further shorten the process of converting a retinal video.
Depending on each compression level, the conversion time for a retinal video from an uncompressed format (500 MB) requires 16–25 s using a 32-bit Windows XP (2.5 RAM, Intel Xeon X5550 Processor 2.67 GHz with NVIDIA Quadro FX 580 graphics card) (Table 3). This timing may vary slightly on different computers with different performance. This is a rapid conversion process as nearly 250 retinal videos can be converted within an hour. As the retinal videos can be readily compressible to be interpreted and stored, the retinal video recording technique can be potentially utilized as a tele-ophthalmology screening tool. It will be of great value to conduct further research to evaluate the cost and clinical effectiveness of performing tele-retinal video recording for DR screening in the remote underserved areas.
In conclusion, a retinal video recording can be compressed from 500 to 20 MB while maintaining its quality, sensitivity, and specificity of DR grading. As network bandwidth is an issue in most of the rural and remote locations, the retinal video transmission speed could be increased by reducing the file size without significant loss of diagnostic information using efficient compression techniques. Given that, the retinal videos are easily compressible while retaining excellent diagnostic accuracy, retinal video recording may be used as an alternative technique in DR screening centers. As this technique is still in its infancy, more research is required to improve the usability of this technique.

References
Williams R, Airey M, Baxter H, Forrester J, Kennedy-Martin T, Girach A et al. Epidemiology of diabetic retinopathy and macular oedema: a systematic review. Eye 2004; 18: 963–983.
Mohamed Q, Gillies MC, Wong TY . Management of diabetic retinopathy: a systematic review. JAMA 2007; 298: 902–916.
Ferris FL 3rd . How effective are treatments for diabetic retinopathy? JAMA 1993; 269: 1290–1291..
Ting DSW, Tay-Kearney ML, Constable IJ, Lim L, Preen DB, Kanagasingam Y et al. Retinal video recording: a new way to image and diagnose diabetic retinopathy. Ophthalmology 2011; 118: 1588–1593.
Newsom RS, Clover A, Costen MT, Sadler J, Newton J, Luff AJ et al. Effect of digital image compression on screening for diabetic retinopathy. Br J Ophthalmol 2001; 85: 799–802.
Eikelboom RH, Yogesan K, Barry CJ, Constable IJ, Tay-Kearney ML, Jitskaia L et al. Methods and limits of digital image compression of retinal images for telemedicine. Invest Ophthalmol Vis Sci 2000; 41: 1916–1924.
Conrath J, Erginay A, Giorgi R, Lecleire-Collet A, Vicaut E, Klein JC et al. Evaluation of the effect of JPEG and JPEG2000 image compression on the detection of diabetic retinopathy. Eye 2007; 21: 487–493.
Wilkinson CP, Ferris 3rd FL, Klein RE, Lee PP, Agardh CD, Davis M et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003; 110: 1677–1682.
Altman DG . Practical Statistics for Medical Reserach. Chapman and Hall: London, UK, 1991.
Acknowledgements
Diabetes Australia Research Trust and Royal Perth Hospital Medical Research Foundation provided the research funding for this study. The sponsor or funding organization had no role in the design or conduct of this research.
Authors contributions
DSW Ting contributed to the study conception and design, data acquisition, data analysis, data interpretation and drafting the article. ML Tay-Kearney contributed to the study conception and design, revision of the important intellectual content, and final approval of the version to be published. J Vignarajan contributed to the data acquisition and data analysis. Y Kanagasingam contributed to the study conception and design, revision of the important intellectual content, and final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ting, D., Tay-Kearney, M., Constable, I. et al. Retinal video recordings at different compression levels: a novel video-based imaging technology for diabetic retinopathy screening. Eye 27, 848–853 (2013). https://doi.org/10.1038/eye.2013.53
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2013.53