Abstract
Purpose
To investigate clinical characteristics and progression rates of the initial central scotomas (ICS) compared with the initial peripheral scotomas (IPS) in normal-tension glaucoma (NTG) patients.
Methods
Among NTG patients showing a single hemifield defect and who performed more than five reliable standard visual field (VF) tests, medical records of ICS (involvement of ≥3 adjacent points with P<5% within the central 12° of fixation and one point with a P<0.01 within the central 6° of fixation) (n=32) or IPS (no VF abnormality within the central 6° of fixation) (n=34) were retrospectively analyzed. The changes of mean thresholds from 10 zones of the glaucoma hemifield test, central 6°and 12° zones, peripheral zones other than central 6° and 12°, and the entire hemifield were inspected. To calculate the progression rates, linear mixed-effect model was employed.
Results
There were no significant differences between the two groups in age, gender, ocular factors including baseline/mean treated intraocular pressure, and systemic factors including systolic or diastolic blood pressure/perfusion pressure, mean ocular perfusion pressure (all P>0.05). There were no significant differences in baseline mean deviation and pattern standard deviation (P>0.05) between the two groups, but VF index was significantly lower in ICS group than in IPS group (P=0.001). The progression rates between the two groups were not significantly different in all zones we investigated (all P>0.05).
Conclusions
Newly diagnosed cases of NTG with ICS may not differ from those with IPS in clinical characteristics and progression rates under treatment.
Similar content being viewed by others
Introduction
The primary purpose of treatment for glaucoma is to maintain the patient’s quality of life through the preservation of vision. From the perspective of quality of life and quality of vision, visual field (VF) defects at or near fixation are more of concern. Patients with VF defects within 5° of fixation are at a greater risk of losing their visual acuity.1 Central VF defects may cause reading difficulty2 and worsen driving performance.3 Therefore, it is important to recognize the progression rates of central VF defects compared with peripheral VF defects in the treatment of glaucoma.
Normal-tension glaucoma (NTG) is a multifactorial disorder with intraocular pressure (IOP) within the statistically normal range.4 Although IOP is part of the pathogenic process in NTG,5, 6, 7 several other factors8, 9, 10, 11, 12, 13 may contribute to optic nerve damage or may make the nerve more susceptible to damage at lower IOP levels.14 For instance, it has been proposed that the average blood flow is decreased and that vascular dysregulation may be a risk factor particularly for patients with NTG.8, 9, 10, 11, 12, 13 VF loss in NTG is reported to be closer to central fixation than in the case of high-tension glaucoma.15, 16, 17 Initial VF defects in NTG can be divided into either central scotomas or peripheral scotomas. On the basis of such findings, different or additional factors may affect the development of these two distinct patterns of VF loss in NTG and may also influence the progression rates.
The purpose of this study was to determine whether we could find the differences in the risk factors and progression rates for initial central scotomas (ICS) and initial peripheral scotomas (IPS) in patients with NTG. We evaluated eyes with either ICS or IPS in a single hemifield and investigated the clinical characteristics and progression rates of these two patterns of VF defects in the early stage of NTG patients.
Materials and methods
Study subjects
Inclusion criteria
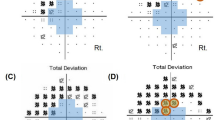
In a retrospective study involving the medical records of 219 patients who were diagnosed with NTG and had more than five VF tests at the Department of Ophthalmology of the Samsung Medical Center, 66 subjects (66 eyes) were selected for the study based on the inclusion criteria described below. Those who showed initial central or peripheral VF defects in either superior or inferior single hemifield defects were included. Each patient was followed up regularly in the outpatient clinic and had a VF test at least once yearly. A diagnosis of NTG was made when a patient with an IOP of ≤21 mm Hg without treatment had findings of glaucomatous optic disc damage and corresponding VF defects, an open angle observed by gonioscopic examination, and no underlying cause for optic disc damage aside from glaucoma. Superotemporal or inferotemporal RNFL defect corresponding to inferior or superior VF defect was identified to make sure only glaucomatous VF defect was included. The central 30-2, SITA-standard strategy program of the Humphrey Field Analyzer (HFA Model640; Humphrey Instruments, Inc., San Leandro, CA, USA) was used for VF testing. A reliable VF fulfilled 3 criteria: fixation loss less than 20%, and false-positive and false-negative rates less than 15%. ICS was defined as the following: (1) involvement of three or more adjacent points with a P<0.05 within the central 12° of fixation; (2) at least one point with a P<0.01 within the central 6° of fixation on the total deviation (TD) plot (Figure 1a). IPS was defined as the following: (1) three or more adjacent points with a P<0.05 outside central 6° of fixation and at least one with P<0.01; (2) no VF abnormality within the central 6° of fixation on the TD plot. (Figure 1b). The definition of a single hemifield defect included the following: (1) three or more adjacent points with a P<0.05 and at least one with a P<0.01 or lesser on the TD probability map, in the superior or inferior hemifield; (2) no points with a P<0.01 or lesser on the TD probability map of the opposite hemifield; (3) a glaucoma hemifield test (GHT) result that is outside the normal limits. If both eyes met the inclusion criteria, the one with the smaller MD was chosen.
The present study was approved by the Institutional Review Board of Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea and followed the tenets of the Declaration of Helsinki.
Exclusion criteria
We excluded patients with (1) lens opacities more severe than C2,N2 andP2 according to the lens opacities classification system III criteria,18 (2) best corrected visual acuity of less than 20/40, (3) spherical equivalent <−6.0 diopters or >+6.0 diopters, (4) initial MD of −20 dB or less, for whom glaucoma progression would be difficult to evaluate using a VF test, (5) non-glaucomatous secondary causes affecting the VF (eg, diabetic retinopathy, other diseases of the optic nerve or retina), (6) other intraocular diseases or neurological diseases affecting the VF (eg, pituitary lesions, demyelinating diseases and intracranial abnormalities), or (7) a history of laser treatment or intraocular surgery (except for uncomplicated cataract surgery).
Treatment
When NTG was diagnosed, the patients received 5 mg/ml betaxolol (Alcon, Inc, Fort Worth, TX, USA) twice daily. During the follow-up, if glaucoma still progressed or did not reach the target IOP, betaxolol was replaced by 50 μg/ml latanoprost (Pfizer, Inc., New York, NY, USA). The first target of IOP reduction was to reduce basal IOP by 20%. However, if the first target IOP was not reached, 16 mm Hg was set as the second target IOP. If latanoprost did not have a sufficient effect, 2 mg/ml brimonidine ml (Allergan, Inc., Irvine, CA, USA) was usually added. The treatment strategy and the follow-up interval were identical in all subjects. A Goldmann applanation tonometer (Haag Streit AG, Bern, Switzerland) was used for IOP measurement. The baseline IOP was calculated as the mean IOP before administration of a topical hypotensive agent at each hospital visit. The post-therapeutic IOP was calculated as the mean IOP after administration of a topical hypotensive agent at each hospital visit.
Clinical data
The prevalence of systemic factors such as diabetes mellitus, systemic hypertension, migraine, orthostatic hypotension, and peripheral vascular disease was evaluated using the questionnaire obtained at the initial visit of each patient and was analyzed to find the systemic risk factors. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in each patient. Systolic perfusion pressure (SPP), diastolic perfusion pressure (DPP), and mean ocular perfusion pressure (MOPP) were calculated according to the following formulae using initial IOP: SPP=SBP−IOP; DPP=DBP−IOP; MOPP=mean BP−IOP; mean BP=DBP+(1/3 × [SBP−DBP]).
Analysis of VF
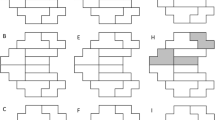
The mean thresholds of the 10 zones corresponding to those of the GHT (Figure 1c), central 6° zone, central 12° zone, peripheral zone other than the central 6° zone, peripheral zone other than the central 12° zone, and the entire hemifield zone based on glaucoma change probability maps of TD were collected from each follow-up VF test. All follow-up results of the VF tests were compared with an average of two baseline VF tests from the same eye. The changes of mean thresholds from the baseline mean thresholds of each designated zone were analyzed to calculate the progression rate. Initial MD, initial pattern standard deviation (PSD), and initial VF index (VFI) were estimated as the average values from the two baseline VF tests.
Statistical analysis
All clinical characteristics were compared between the ICS group and the IPS group using independent t test, χ2-test, or Fisher’s exact test. P<0.05 was considered significant.
To calculate the progression rate, which is the slope of the mean thresholds of each zone, the linear mixed-effect model with unequal random effect variances was employed. P<0.005 for the slope of 10 zones, P<0.0125 for the slope of central or peripheral zones, and P<0.025 for the slope of the entire hemifield zones were considered to be statistically significant.19, 20 Only such significant values were selected for the calculation of the mean progression rates. Covariates such as age, gender, initial IOP, initial MD, initial PSD, and initial VFI were controlled. The progression rates of each zone between the ICS group and the IPS group were also compared using the linear mixed-effect model. P<0.005 and 99.5% CI for the 10 zones, P<0.0125 and 98.75% CI for the central or peripheral zones, and P<0.025 and 97.5% CI for the entire hemifield zones were considered to be statistically significant.19, 20 All statistical analyses were conducted using SAS software version 9.1.3 (SAS, Inc., Cary, NC, USA) and SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Baseline characteristics
Clinical characteristics
Among the 66 eligible patients who met the inclusion criteria, 32 subjects (32 eyes) were included in the ICS group, and 34 subjects (34 eyes) were included in the IPS group. The mean follow-up period was 7.34±2.29 years overall. Clinical characteristics of the two groups are shown in Table 1. There were no significant differences in the follow-up period, mean age at first visit, gender, and family history of glaucoma between the two groups (all P>0.05). Systemic factors including diabetes mellitus, hypertension, migraine, orthostatic hypotension, and peripheral vascular disease showed no significant difference between the two groups (all P>0.05). Ocular factors including spherical equivalent, central corneal thickness, disc hemorrhage (DH) detection, and presence of β-zone peripapillary atrophy also showed no significant difference between the two groups (all P>0.05). No significant differences were found in baseline IOP and post-therapeutic mean IOP between the two groups (all P>0.05) (Table 1).
There were no significant differences in the therapeutic classes between the two groups. (all P>0.05) (Table 2). Three patients from ICS group and four patients from IPS group underwent uncomplicated cataract surgery.
BP-related parameters are demonstrated in Table 3. There was no significant difference in SBP or DBP between the two groups (P=0.510, P=0.088, respectively). Calculated SPP and DPP also showed no significant difference between the two groups (P=0.510, P=0.088, respectively). MOPP was 91.03±10.24 in the ICS group and 94.91±10.36 in the IPS group. No significant difference was found in MOPP between the two groups (P=0.131) (Table 3).
Perimetric parameters
The mean number of VF tests was 7.52±2.46 in total. Initial MD was −2.29±1.67 in the ICS group and −2.16±1.87 in the IPS group. Initial PSD was 5.74±3.09 in the ICS group and 4.62±2.63 in the IPS group. There was no significant difference in initial MD or initial PSD between the two groups (P=0.765, P=0.117, respectively). Initial VFI was 90.74±6.13 in the ICS group and 95.37±4.03 in the IPS group. Initial VFI in ICS group was significantly lower than that of the IPS group (P=0.001). Involvement of the superior hemifield was 81% (26/32) in the ICS group and 50% (17/34) in the IPS group. Superior hemifield defect was significantly more predominant in the ICS group than in the IPS group (P=0.008) (Table 3).
Progression rates of each zone in ICS group and IPS group
Progression rates were calculated as regression coefficients from the linear mixed-effect model. Progression rates from the 10 zones of the GHT and from the central or peripheral field, and the entire hemifield (according to the initially affected hemifield) are shown in Table 4. There were no significant differences in the progression rates between the two groups in all 10 zones of the GHT (P=0.539, P=0.496, P=0.455, P=0.306, P=0.988, respectively).
There were no significant differences in the progression rates between the two groups in the designated central or peripheral field (P=0.151, P=0.908, P=0.845, P=0.767, respectively). Progression rates from the entire hemifield (according to the initially affected hemifield) in the ICS group and the IPS group were −0.606 dB/year and −0.565 dB/year, respectively. There was also no significant difference in the progression rates from the entire hemifield between the two groups (P=0.745) (Table 4).
Progression rates from all of the zones we investigated showed no significant differences between the ICS group and the IPS group.
Discussion
In the present study, we compared the clinical characteristics including BP-related parameters and progression rates between the ICS group and the IPS group in Korean NTG patients. These two groups had similar initial MD, PSDs and also similar baseline and post-therapeutic mean IOPs, and we followed them for up to 10 years. We intended to determine if there was any difference in clinical characteristics or progression rates between the two groups. Contrary to our expectations, there were no differences in the clinical characteristics between the two groups except for the initial VFI and the occurrence of superior hemifield.
The VFI is the aggregate percentage of visual function for a given field at each point where visual thresholds are estimated. The percentage of visual loss at each point is calculated on the basis of pattern deviations up to −20 dB and TDs beyond −20 dB. Central points have more weight than peripheral points.21 Therefore, the ICS group had significantly lower initial VFI than that of the IPS group when the initial MD and PSD were similar between the two groups.
Glaucomatous VF defects are often identified in the upper field, both in primary open-angle glaucoma (POAG) and NTG.22, 23, 24, 25 Interestingly, superior VF defects were significantly more identified in the ICS group (81%) than in the IPS group (50%) in our study. These results correspond with a previous study. Park et al26 reported that parafoveal scotomas occurred predominantly in the superior hemifield in both NTG (79%) and high pressure glaucoma (87%) subgroups. It seems that glaucomatous central VF defects can be observed more in the superior hemifield than in the inferior hemifield.
It has been reported that, with progression, NTG in the early stage trends toward local depression, particularly in the upper central and upper nasal VFs.27 Ahrlich et al28 showed that NTG eyes progress more often in the central VF. Although early stage NTG may progress more often in the central VF, it is noticeable that the ‘rate’ of progression, which was the slope of the mean thresholds that also reflected the deepening of the VF defect, was not significantly different between the ICS group and the IPS group in all zones we investigated.
ICS group had scotomas mainly in the central region, and IPS group had scotomas other than the central region in the beginning. During the 7.34±2.29 years of follow-up period, 20/34 (58.8%) patients from the IPS group had involvement of three or more of adjacent points with P<5% within the central 12° of fixation at the final VF test. ICS from the ICS group tended to spread toward the peripheral region. Therefore, when we compared the progression rates in both central and peripheral zones (GHT zones, central 6°and 12° zones, peripheral zones other than central 6° and 12°, and the entire hemifield), the rates were not significantly different between the two groups. Our results indicate that the ICS spread out toward the periphery, and the IPS expand toward the center at a similar progression rate.
According to our inclusion criteria, the two groups had some gray zones. Overlapping zone was identified as one or two points near or the outermost central 12° zone (8 points in each hemifield) in five patients from IPS group. However, in those patients, the central 6° zones were cleared and the whole pattern of VF defect was nasal step or arcuate scotomas. We believe it is not unreasonable to apply these inclusion criteria if the purpose is to clinically compare the patients showing mainly central or mainly peripheral VF defects we encounter in practice. Even though there were minor overlapping points between the two groups, we think that it did not entirely affect the rate of progression in ‘all’ the zones we investigated.
From the perspective of quality of life and quality of vision, VF defects at or near fixation are more of concern. However, our results suggest that the central VF defects do not progress any faster than the peripheral VF defects. This finding may serve as a relief in the treatment of NTG patients involving central VF, especially when they are under proper hypotensive treatment.
Higher frequency of DH detection, systemic hypotension, migraine, and Raynaud’s phenomenon or peripheral vascular disease have been reported to be the IOP-independent risk factors for glaucoma, especially for NTG.29, 30, 31, 32, 33, 34, 35, 36, 37, 38 Park et al26 demonstrated that DH was detected more often in the initial parafoveal scotoma group than in initial nasal step group in the subgroup of normal pressure glaucoma. However, in the present study, such findings were not observed. When the frequency of DH is compared with that of previous reports, the follow-up period and interval between visits should be considered.26 Although the mean interval between visits was 4.1–4.9 months in the Park’s study, the follow-up interval was 6 months in our study. There may be a possibility of missed DH detection between visits, which may have affected our results.
Many studies have suggested that impaired autoregulation in ocular blood flow (OBF) may be present in POAG.39, 40, 41, 42, 43, 44 In NTG, it has been reported that 24-h OPP instability correlates with the severity of glaucomatous VFs at initial presentation.32, 39 24-h unstable OPP was also reported to be strongly associated with glaucomatous VF progression in NTG patients.40 A previous study showed that systemic hypotension was significantly more prevalent in the initial parafoveal scotoma group than in the initial nasal step group in the subgroup of normal pressure glaucoma.26 Systemic hypotension is considered to be related with impaired OBF. However, in our study, SBP, DBP, SPP, DPP, and MOPP were lower in the ICS group than that of the IPS group, but the differences were not statistically significant. A single measurement of BP was performed at the initial visit, and MOPP was calculated on the basis of the theoretical formula rather than from a direct measurement of OBF. Continuous monitoring of BP or MOPP in a physiologic condition could have led to different results. However, currently there are no available direct methods to measure MOPP in a physiologic condition. Further study and methodology on this matter will be necessary for a proper understanding of the role of ocular perfusion on NTG, especially on central VF defects.
One of the strengths of this study is that it analyzed a cohort of NTG patients who were followed for a relatively long duration of 7.34±2.29 years by a single physician. We directly investigated the rate of progression by measuring changes in the mean thresholds of the designated zones, including the GHT zones, central and peripheral zones, and the entire hemifield, instead of merely using MD indexes which were reported to be insufficient to identify progression in slowly progressing NTG patients.45 The subjects in our study had similar baseline MD and PSD between two groups; this strengthens the statistical power of the study for comparison between groups and excludes other possible perimetric index factors that could affect the progression.46 The two groups had not only similar initial IOP but also the mean post-treatment IOP. This also excludes the possible influence of IOP to baseline clinical characteristics or progression rates.
Several limitations of the present study should be acknowledged mainly due to its retrospective nature. Inclusion of patients who were followed up for a relatively long period of time could have affected the accuracy of the estimated proportions. The relatively small sample size should also be taken into consideration. A number of patients were excluded from the analysis because of missing data at the initial visit, including information about systemic factors, and the effect of this selection of patients on our results being not known. However, to compare the clinical characteristics and to calculate the progression, patients with full clinical information and more than five VF tests were required. Also, they had to fulfill the ICS or IPS criteria. Moreover, we cannot rule out the possibility that our results were confounded by other risk factors that we have not evaluated. We evaluated relatively early stage NTG patients (mean MD=−2.22±1.77 dB) with hypotensive treatment. However, this finding regarding progression rates may serve as an important reference to clinicians in the treatment of NTG, which may show more central VF defect, especially in the early stage.15, 16, 17
In conclusion, ICS vs IPS may not differ in clinical characteristics, other than VFI and the occurrence of superior hemifield, as well as progression rates in the early stage of NTG patients under treatment. A large, multicenter randomized clinical trial is required to draw more definite conclusions.

References
Kolker AE . Visual prognosis in advanced glaucoma: a comparison of medical and surgical therapy for retention of vision in 101 eyes with advanced glaucoma. Trans Am Ophthalmol Soc 1977; 75: 539–555.
Fujita K, Yasuda N, Oda K, Yuzawa M . [Reading performance in patients with central visual field disturbance due to glaucoma]. Nihon Ganka Gakkai Zasshi 2006; 110: 914–918.
Coeckelbergh TR, Brouwer WH, Cornelissen FW, Van Wolffelaar P, Kooijman AC . The effect of visual field defects on driving performance: a driving simulator study. Arch Ophthalmol 2002; 120: 1509–1516.
Levene RZ . Low tension glaucoma: a critical review and new material. Surv Ophthalmol 1980; 24: 621–664.
Bito LZ . Impact of intraocular pressure on venous outflow from the globe: a hypothesis regarding IOP-dependent vascular damage in normal-tension and hypertensive glaucoma. J Glaucoma 1996; 5: 127–134.
Schulzer M . Intraocular pressure reduction in normal-tension glaucoma patients. The Normal Tension Glaucoma Study Group. Ophthalmology 1992; 99: 1468–1470.
Cartwright MJ, Anderson DR . Correlation of asymmetric damage with asymmetric intraocular pressure in normal-tension glaucoma (low-tension glaucoma). Arch Ophthalmol 1988; 106: 898–900.
Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R, Varotto A . Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology 2000; 107: 1287–1293.
Bresson-Dumont H, Bechetoille A . [Role of arterial blood pressure in the development of glaucomatous lesions]. J Fr Ophtalmol 1996; 19: 435–442.
Cioffi GA, Sullivan P . The effect of chronic ischemia on the primate optic nerve. Eur J Ophthalmol 1999; 9 (Suppl 1): S34–S36.
Flammer J, Orgul S . Optic nerve blood-flow abnormalities in glaucoma. Prog Retin Eye Res 1998; 17: 267–289.
Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 2002; 21: 359–393.
Fuchsjager-Mayrl G, Wally B, Georgopoulos M, Rainer G, Kircher K, Buehl W et al. Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci 2004; 45: 834–839.
Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 1998; 126: 487–497.
Caprioli J, Spaeth GL . Comparison of visual field defects in the low-tension glaucomas with those in the high-tension glaucomas. Am J Ophthalmol 1984; 97: 730–737.
Chauhan BC, Drance SM, Douglas GR, Johnson CA . Visual field damage in normal-tension and high-tension glaucoma. Am J Ophthalmol 1989; 108: 636–642.
Thonginnetra O, Greenstein VC, Chu D, Liebmann JM, Ritch R, Hood DC . Normal versus high tension glaucoma: a comparison of functional and structural defects. J Glaucoma 2010; 19: 151–157.
Chylack LT Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 1993; 111: 831–836.
Smith SD, Katz J, Quigley HA . Analysis of progressive change in automated visual fields in glaucoma. Invest Ophthalmol Vis Sci 1996; 37: 1419–1428.
Katz J, Gilbert D, Quigley HA, Sommer A . Estimating progression of visual field loss in glaucoma. Ophthalmology 1997; 104: 1017–1025.
Bengtsson B, Heijl A . A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol 2008; 145: 343–353.
Lewis RA, Hayreh SS, Phelps CD . Optic disk and visual field correlations in primary open-angle and low-tension glaucoma. Am J Ophthalmol 1983; 96: 148–152.
Grave EL, Geijssen C . Comparison of glaucomatous visual field defects in patients with high and low intraocular pressures. In: Greve EL, Heijl A (eds) Fifth International Visual Field Symposium: Sacramento, October 20-23, 1982 5th ed. Kluwer: Boston, MA, USA, 1983 pp 101–105.
Anderson DR, Hitchings R . A comparative study of visual field of patients with low-tension glaucoma and those with chronic simple glaucoma. In: Greve EL, Heijl A (eds) Fifth International Visual Field Symposium: Sacramento, October 20-23, 1982. Kluwer: Boston, MA, USA, 1983 pp 97–99.
Cantor L . Achieving low target pressures with today’s glaucoma medications. Surv Ophthalmol 2003; 48 (Suppl 1): S8–16.
Park SC, De Moraes CG, Teng CC, Tello C, Liebmann JM, Ritch R . Initial parafoveal versus peripheral scotomas in glaucoma: risk factors and visual field characteristics. Ophthalmology 2011; 118: 1782–1789.
Fukuchi T, Yoshino T, Sawada H, Seki M, Togano T, Tanaka T et al. Progression rate of total, and upper and lower visual field defects in open-angle glaucoma patients. Clin Ophthalmol 2010; 4: 1315–1323.
Ahrlich KG, De Moraes CG, Teng CC, Prata TS, Tello C, Ritch R et al. Visual field progression differences between normal-tension and exfoliative high-tension glaucoma. Invest Ophthalmol Vis Sci 2010; 51: 1458–1463.
Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL . Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol 1994; 117: 603–624.
Demailly P, Cambien F, Plouin PF, Baron P, Chevallier B . Do patients with low tension glaucoma have particular cardiovascular characteristics? Ophthalmologica 1984; 188: 65–75.
Graham SL, Drance SM . Nocturnal hypotension: role in glaucoma progression. Surv Ophthalmol 1999; 43 (Suppl 1): S10–S16.
Choi J, Jeong J, Cho HS, Kook MS . Effect of nocturnal blood pressure reduction on circadian fluctuation of mean ocular perfusion pressure: a risk factor for normal tension glaucoma. Invest Ophthalmol Vis Sci 2006; 47: 831–836.
Corbett JJ, Phelps CD, Eslinger P . Montague PR. The neurologic evaluation of patients with low-tension glaucoma. Invest Ophthalmol Vis Sci 1985; 26: 1101–1104.
Phelps CD, Corbett JJ . Migraine and low-tension glaucoma. A case-control study. Invest Ophthalmol Vis Sci 1985; 26: 1105–1108.
Drance S, Anderson DR, Schulzer M . Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol 2001; 131: 699–708.
Flammer J, Guthauser U, Mahler F . Do ocular vasospasms help cause low-tension glaucoma? Doc Ophthalmol Proc Ser 1987; 49: 397–399.
Drance SM, Douglas GR, Wijsman K, Schulzer M, Britton RJ . Response of blood flow to warm and cold in normal and low-tension glaucoma patients. Am J Ophthalmol 1988; 105: 35–39.
Broadway DC, Drance SM . Glaucoma and vasospasm. Br J Ophthalmol 1998; 82: 862–870.
Choi J, Kim KH, Jeong J, Cho HS, Lee CH, Kook MS . Circadian fluctuation of mean ocular perfusion pressure is a consistent risk factor for normal-tension glaucoma. Invest Ophthalmol Vis Sci 2007; 48: 104–111.
Sung KR, Lee S, Park SB, Choi J, Kim ST, Yun SC et al. Twenty-four hour ocular perfusion pressure fluctuation and risk of normal-tension glaucoma progression. Invest Ophthalmol Vis Sci 2009; 50: 5266–5274.
Sung KR, Cho JW, Lee S, Yun SC, Choi J, Na JH et al. Characteristics of visual field progression in medically treated normal-tension glaucoma patients with unstable ocular perfusion pressure. Invest Ophthalmol Vis Sci 2011; 52: 737–743.
Hafez AS, Bizzarro R, Descovich D, Lesk MR . Correlation between finger blood flow and changes in optic nerve head blood flow following therapeutic intraocular pressure reduction. J Glaucoma 2005; 14: 448–454.
Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC . Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch Ophthalmol 1995; 113: 216–221.
Hafez AS, Bizzarro RL, Rivard M, Lesk MR . Changes in optic nerve head blood flow after therapeutic intraocular pressure reduction in glaucoma patients and ocular hypertensives. Ophthalmology 2003; 110: 201–210.
Anderson DR, Drance SM, Schulzer M . Natural history of normal-tension glaucoma. Ophthalmology 2001; 108: 247–253.
Rao HL, Kumar AU, Babu JG, Senthil S, Garudadri CS . Relationship between severity of visual field loss at presentation and rate of visual field progression in glaucoma. Ophthalmology 2011; 118: 249–253.
Acknowledgements
An exemption from informed consent for research was granted because this was a retrospective research study.
Disclaimer
The authors specific contributions to the work described herein are: design of the study (CK); conduct of the study (HC, JL, ML, CK); data collection and management, analysis and interpretation of the data (HC, JL, ML, CK); preparation, review, and approval of the manuscript (HC, CK). The present study was approved by the Institutional Review Board of Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cho, Hk., Lee, J., Lee, M. et al. Initial central scotomas vs peripheral scotomas in normal-tension glaucoma: clinical characteristics and progression rates. Eye 28, 303–311 (2014). https://doi.org/10.1038/eye.2013.285
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2013.285
Keywords
This article is cited by
-
Deep learning visual field global index prediction with optical coherence tomography parameters in glaucoma patients
Scientific Reports (2023)
-
MicroRNA profiles in aqueous humor between pseudoexfoliation glaucoma and normal tension glaucoma patients in a Korean population
Scientific Reports (2022)
-
Determinants of vessel defects in superficial and deep vascular layers in normal-tension glaucoma using optical coherence tomography angiography
Scientific Reports (2021)
-
Deep learning classification of early normal-tension glaucoma and glaucoma suspects using Bruch’s membrane opening-minimum rim width and RNFL
Scientific Reports (2020)
-
Normal tension glaucoma: review of current understanding and mechanisms of the pathogenesis
Eye (2018)