Abstract
Purpose
To investigate the changes in endothelial cell count, central corneal thickness (CCT), and refractive error after a session of selective laser trabeculoplasty (SLT) for open angle glaucoma (OAG).
Methods
This prospective cohort study recruited 111 eyes of 66 consecutive subjects with OAG. Subjects received SLT to 360° of the trabecular meshwork. Endothelial cell count, CCT, and spherical equivalent were measured at baseline before SLT as well as at 1 week and 1 month post SLT. A repeated measure nested ANOVA with Tukey’s multiple comparison test was performed to compare the outcome measures before and after SLT.
Results
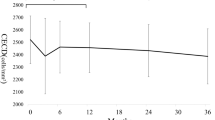
In 111 eyes of 66 subjects, the mean number of laser applications per treatment was 166.9±41.4 with a mean energy level of 1.0±0.07 mJ. The mean endothelial cell count decreased significantly from 2465.0±334.0 cells/mm2 at baseline to 2355.0±387.0 cells/mm2 at 1 week (P=0.0004) but increased to baseline levels at 1 month post SLT (2424.0±379.4 cells/mm2, P=0.3). The CCT, which decreased from a baseline of 549.4±37.6 to 543.9±40.2 μm at 1 week post SLT (P=0.02), also returned to the baseline level by 1 month (P=0.2). The spherical equivalent was static from baseline. A positive correlation was found between total laser energy and CCT at 1 month post treatment (r=0.3, P=0.005).
Conclusion
The transient reductions in endothelial cell count and CCT following SLT returned to baseline levels 1 month after the procedure. Patients undergoing SLT should be aware of the risk of potential corneal changes.
Similar content being viewed by others
Introduction
Selective laser trabeculplasty (SLT, Lumenis, Yokneam, Israel) was approved by the United States Food and Drug Administration in 2001 for the treatment of open-angle glaucoma (OAG) following which there was a doubling, over the subsequent three years, of the number of laser trabeculoplasty procedures performed annually.1 The rapid increase in SLT procedures following its introduction was largely attributed to similar effectiveness relative to argon laser trabeculoplasty (ALT) but with fewer adverse side effects. The lack of visible trabecular meshwork scarring following SLT led to a more frequent re-treatment with this procedure relative to ALT.2 SLT has been established to be a safe procedure with few known side effects including post-procedure intraocular pressure (IOP) elevation, blurred vision, ocular irritation and iritis, all of which are generally transient, lasting no more than a few days.3, 4, 5, 6, 7, 8
Permanent corneal changes after SLT are extremely rare with only two reported cases9 in the literature of corneal edema, haze, and thinning occurring within 24–48 h post laser. These cases resulted in irreversible corneal scarring, thinning, and a myopic shift in refractive error. In one of the cases, the patient was on long-term topical cyclosporine before SLT and tested positive for serum antibodies to Herpes simplex virus-1; thus, the herpetic keratitis may have been a confounding variable with regard to the clinical findings.9 Given that the trabecular meshwork is in close proximity to the cornea, one can postulate that there may be damage to the cornea with SLT that is not clinically evident. This is one of the few studies in the literature measuring endothelial cell counts, central corneal thickness (CCT), and refractive error before and after SLT.
Patients and methods
This study adhered to the tenets of the Declaration of Helsinki. Informed patient consent and approval by the Institutional Review Board were obtained before study commencement. All subjects were recruited sequentially from the ophthalmology clinic of a tertiary university hospital (Queen Mary Hospital) in Hong Kong during September 2011 to September 2012. The study included subjects with unilateral or bilateral primary open-angle or normal tension glaucoma who were receiving IOP-lowering medications at the time of the study. Subjects were excluded if they had pre-existing corneal pathology or scars, previous SLT treatment, or if they had been lost to follow-up. SLT was offered to subjects as an adjuvant IOP-lowering therapy with the aim of reducing their anti-glaucoma medication requirements.
Baseline IOP was measured followed by a single session of SLT performed by one surgeon (JWL) using a Q-switched Nd:YAG laser (Ellex Medical Lasers, Adelaide, SA, Australia) for 360° in all patients. An initial energy of 0.8 mJ was used with titration in energy until bubble formation was just visible in the trabecular meshwork. Subjects with bilateral disease were offered treatment of both eyes in the same session. A Latina SLT gonio laser lens was used for treatment after disinfection by Presept 0.5 g disinfection tablets (Johnson & Johnson, New Brunswick, NJ, USA) dissolved in normal saline. In all treated eyes, a single drop of Alphagan P (Allergan Inc, Waco, TX, USA) was instilled immediately post SLT and a Dexamethasone 0.1% and Neomycin 0.5% combination eye drop (Dexoptic-N by Ashford Laboratories Pvt. Ltd., Mumbai, India) was used twice daily for 1 day and subsequently continued for 2–3 days if anterior chamber reaction was seen on the day following SLT. Patients returned for follow-up 1 day, 1 week, and 1 month following SLT. Patients continued the same pre-SLT topical anti-glaucoma medication regime throughout the duration of the study.
The endothelial cell count was calculated using non-contact specular microscopy (Noncon ROBO-CA by Konan Medical USA Inc., Irvine, CA, USA), the CCT obtained via videokeratography (Orbscan IIz by Bausch & Lomb, Rochester, NY, USA), and the spherical equivalent computed via kerato-refractometer (Topcon KR-8900 by Topcon Europe Medical B.V., Capelle a/d Ijssel, The Netherlands). All parameters were measured by a trained optometrist at baseline before SLT, as well as 1 week and 1 month after SLT.
Baseline retinal nerve fiber layer thickness via a Spectralis Optical Coherence Tomography (Heidelberg Engineering, Carlsbad, CA, USA) and visual field via a Humphrey Visual Field Analyzer (Carl Zeiss Meditec AG, Berlin, Germany) 24-2 STIA protocol were recorded.
Measurement of endothelial cell count
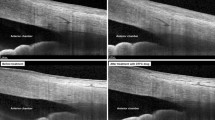
The central corneal endothelium was photographed using a non-contact specular microscope. The patient’s head was stabilized on the specular microscope with the chin and forehead rests. The microscope was aligned and focused on the center of the cornea by an experienced optometrist. The automatic function of the machine captured the central endothelium (field size of 0.25 × 0.40 mm, resolution of 100 lines/mm). The central corneal endothelial cell density was assessed and defined as the number of cells per mm2. Adequate tear film was ensured before each scan and the repeatability of measurements was confirmed.
Snellen best-corrected visual acuity (BCVA) was measured at baseline and at 1 month post SLT. All patients underwent a slit lamp examination with IOP measurement at baseline, 1 week, and 1 month after SLT. All IOP measurements were obtained using Goldmann applanation tonometry.
A repeated measure ANOVA with nested design and Tukey’s multiple comparison test was performed to assess for differences between each time point before and following SLT for the following outcome measures: endothelial cell count, CCT, spherical equivalent, and IOP. The nested ANOVA was used to account for using both eyes from patients in the same sample.10 For BCVA, Snellen acuity was converted to LogMAR units and paired t-test was used to assess the differences in means between the pre- and post-SLT BCVA. The total laser energy was calculated by multiplying the number of laser shots with the energy level used for treatment in each case. Pearson correlation was calculated to analyze the association of total laser energy and number of anti-glaucoma medications with each of the following variables: endothelial cell count, CCT, and spherical equivalent. A Friedman test with Dunn’s Multiple Comparison was used to compare for difference between the corneal parameters of the treated vs the untreated fellow eyes in the 11 eyes with unilateral SLT treatment.
Repeatability testing
To validate the reproducibility of endothelial cell count, CCT, spherical equivalent, and BCVA measurements, simultaneous readings from the fellow non-treated eye of 11 subjects receiving unilateral SLT treatment were recorded. A Wilcoxon matched-pairs signed rank test was employed to compare the monocular BCVA at baseline and 1 month in the fellow non-treated eye. The baseline and 1 month VA was 0.07±0.07 and 0.09±0.09 LogMAR units, respectively (P=0.5). For the endothelial cell count, CCT, and spherical equivalent, the intraclass correlation coefficient (ICC) was chosen to compare baseline, 1 week, and 1 month. The average ICC for the three parameters was 0.997 (95% confidence interval: 0.995–0.998), indicating high reproducibility.
Results
Of the 111 eyes of 66 subjects analyzed in the study, there were 53 left eyes and 58 right eyes. The mean age was 63.9±11.9 years old. Forty-three and sixty-eight eyes had been diagnosed with POAG and NTG, respectively. All subjects underwent SLT treatment for the first time and received a single treatment. The baseline IOP in the POAG and NTG groups was 18.0±4.4 and 16.0±2.0 mm Hg, respectively (P=0.002). The mean number of anti-glaucoma eye drops used was 2.0±1.1. There were no significant correlations between the number of anti-glaucoma medications and the corneal parameters (all P>0.6). The mean number of SLT laser applications was 166.9±41.4 with a mean energy level of 1.0±0.07 mJ. The mean total laser energy used was 163.0±43.3 mJ (Table 1).
The mean endothelial cell count was 2465.0±334.0 cells/mm2 at baseline, decreased by 4.5% to 2355±387 cells/mm2 at 1 week and increased back to 2424.0±379.4 cells/mm2 at 1 month post SLT (Table 2). There were statistically significant differences between the baseline vs 1 week endothelial cell count (P=0.0004) as well as the 1 week vs 1 month endothelial cell count (P=0.04). There was, however, no significant difference between the baseline vs 1 month endothelial cell count (P=0.3). There was no correlation between the total laser energy applied and the endothelial cell count at 1 week or 1 month post SLT (P>0.4).
The mean CCT was 549.4±37.6 μm at baseline, 543.9±40.2 μm at 1 week, and 546.2±38.1 μm at 1 month post SLT. There was a statistically significant 1% decrease in CCT at 1 week post-SLT compared with baseline (P=0.02) but there was no significant difference between the CCT at baseline vs 1 month post SLT (P=0.2). There was a small but statistically significant positive correlation between the total laser energy applied and the CCT at 1 month (r=0.3, P=0.005) but not at 1 week (r=0.1, P=0.4).
The mean spherical equivalent was −3.5±4.2 diopters (D) at baseline, −3.4±4.1 D at 1 week, and −3.3±4.2 D at 1 month post SLT. There were no significant differences in the means between the various time intervals (all P>0.2). There was also no correlation between the total laser energy applied and the spherical equivalent at 1 week or 1 month (P>0.8) following SLT treatment. The BCVA at baseline and 1 month post SLT was 0.3±0.3 and 0.2±0.2 LogMAR units, respectively (P<0.0003). In the fellow non-treated eyes of those receiving unilateral SLT, the BCVA at baseline and 1 month was 0.07±0.07 and 0.09±0.09 LogMAR units, respectively (P=0.5).
The baseline pre-treatment IOP for all subjects was 16.7±3.3 mmHg. The 1 day, 1 week, and 1 month post SLT IOP’s were 12.0±2.7 (28% reduction), 15.1±3.6 (9.5% reduction), and 13.2±2.7 mmHg (21.0% reduction), respectively. Tukey’s multiple comparison test showed significant differences between these IOPs at all time intervals (P<0.0001).
There were no complications that were attributed to the SLT procedures. None of the eyes developed clinically visible corneal edema on slit lamp examination. All of the eyes with anterior chamber reaction had complete resolution with regard to this parameter within 5 days following SLT.
In the control group of 11 subjects used for repeatability testing, there were three right eyes and eight left eyes. The mean endothelial cell count was 2646.0±358.0 cells/mm2 at baseline, 2737.0±323.4 cells/mm2 at 1 week, and 2623.0±266.5 cells/mm2 at 1 month. The mean CCT was 573.9±34.8 μm at baseline, 568.6±30.4 μm at 1 week, and 576.4±28.0 μm at 1 month. The mean spherical equivalent was −2.1±1.7 D at baseline, −2.1±1.5 D at 1 week, and −2.2±1.5 D at 1 month. There were no significant differences between the corneal parameters in the control group at any of the time points (all P>0.05). There was no statistical significant difference between the corneal parameters of the treated vs the fellow eyes in these 11 eyes (Table 3). The BCVA of the untreated eye was 0.07±0.07 and 0.09±0.09 LogMAR at baseline and 1 month, respectively (P=0.5).
Discussion
SLT has been shown to have a similar efficacy to ALT but with far less histopathologically confirmed damage to the non-pigmented trabecular meshwork. SLT uses a nanosecond pulse technology that delivers only 1% of the energy used in ALT.11 The reported side effects of SLT are relatively mild and transient, including anterior chamber reaction, IOP elevation, eye pain, non-specific conjunctivitis, corneal edema, blurred vision, and, rarely, the appearance of corneal keratitis or scarring.3, 4, 5
White et al. reported in a series of 10 patients treated with 180° SLT that subtle endothelial changes were diffusely noted on slit lamp examination but these changes disappeared by 6 weeks. The endothelial cell count measured at 1 h after SLT (2237±211 cells/mm2) and 6 weeks after SLT (2278±242 cells/mm2) were comparable and normal.12 In our larger study, we used objective assessments, using specular microscopy, videokeratography, and kerato-refractometer to document corneal changes in the initial month following SLT. In the 111 eyes with OAG treated with a single session of SLT, we noted a transient but significant 5% apparent reduction in endothelial cell count from 2465.0±334.0 cells/mm2 at baseline to 2355±387 cells/mm2 at 1 week and increasing back to 2424.0±379.4 cells/mm2 at 1 month. This transient reduction in cells was noted in the form of dark areas on specular microscopy, possibly due to inflammatory cell attachment on the endothelium or cellular edema separating the endothelial cells from the Descemet’s membrane impairing their count on specular microscopy. Similar transient endothelial change has also been reported as a feature seen with other ocular pathologic conditions, including superficial keratopathy, stromal keratitis, and anterior uveitis, and such changes are usually clinically insignificant unless there are chronic and multiple attacks.13 It is also noteworthy that we did not use alcohol to disinfect the SLT lenses as alcohol has been shown to cause superficial keratopathy and corneal edema, which can potentially confound results on corneal structure and function.13 Presept, which was used for sterilization in our study, is not known to cause keratopathy. Furthermore, the lenses used in performing SLT were rinsed with normal saline after disinfection. As all of the anterior chamber inflammation in our treated eyes subsided by 3–5 days after SLT, it appears the likely cause for the apparent reduction of endothelial cell count is the transient edema at the cellular level obscuring endothelial cell detection during specular microscopy. The reproducibility of the investigations were high with an average ICC for the three parameters being 0.997, thus, it is less likely that the observed changes are due to repeatability of the measurement devices.
The incidence of corneal edema post SLT has been previously reported as being approximately 0.8%.4 Our findings suggest that the incidence of endothelial cell edema may be substantially higher as reflected by the apparent reduction in endothelial cell count at 1 week, with a gradual normalization to pre-laser levels by 1 month after treatment. SLT has also been reported in the treatment of secondary glaucoma after penetrating keratoplasty without any detrimental effect on the corneal graft at 6 months post SLT.14
In our study, the mean IOP on post-SLT day 1 was 12.0±2.7 mmHg, followed by a smaller decrease from baseline at week 1 (15.1±3.6 mmHg) and month 1 (13.2±2.7 mmHg). All such reductions were statistically significantly lower than baseline. Although the effect of SLT may be greatest immediately after treatment15, 16 because of the increase in porosity of the trabecular meshwork from the upregulation of metalloproteinases, cytokines, and macrophages,17 the lower IOP on day 1 post SLT may also be contributed in part to a falsely low IOP reading resulting from alterations in corneal hysteresis from the subclinical corneal edema. It has been demonstrated that corneal edema after phacoemulsification has been associated with a relatively lower IOP by Goldmann applanation tonometry because of the reduction of corneal hysteresis and alterations of the stromal lamellar architecture.18, 19, 20 Another possibility is the use of Alphagan P immediately after SLT but as only a single drop was instilled, it is unlikely to cause a significant drop in day 1 IOP. Thus, the IOP at 1 month post SLT may more accurately reflect the true change in IOP as a result of the SLT procedure. Another possibility for an early IOP reduction might be due to improved medication compliance immediately after SLT followed by self-tapering when the IOP was reduced in subsequent clinical visits.
The CCT was significantly thinned from a mean of 549.4±37.6 μm at baseline to 543.9±40.2 μm at 1 week post SLT. This reduction appeared to be transient as the measurement increased to 546.2±38.1 μm at 1 month post laser without any significant difference in comparison to the baseline value. We postulate that the statistically significant thinning may be due to dissipation of heat from the laser energy into the corneal stroma, resulting in temporary thermal contractions of the stromal collagen fibers, similar to the effect seen with laser thermokeratoplasty. Such findings have been reported after the use of the holmium YAG (Ho:YAG) laser, with contraction of all layers of the corneal stroma in response to thermal coagulation subsequently followed by replenishing of keratocytes to the treated area during the healing process.21 What has been unaccounted for is whether or not the SLT lens used contributes to the changes in CCT. Future studies looking into this aspect would be of interest.
The apparent reduction in endothelial cell count does suggest an endothelial cell edema although this should have resulted in an increase in corneal thickness. In contrast, we noted a thinning of the CCT at 1 week. This can be explained by the postulation that the stromal contractions induced by the laser heat dissipation had a greater effect on the CCT than the endothelial cell edema, causing a paradoxical decrease in CCT. We theorize that stromal remodeling, keratocyte migration, and corneal healing enable the CCT to return to baseline levels by the 1 month post treatment time point.
We further postulate that the interim findings described above seen following SLT are reflective of the endothelial cells remaining healthy, unlike in the case of chronic corneal conditions such as Fuch’s endothelial dystrophy where initial changes in corneal architecture are followed by permanent increases in corneal thickness as endothelial cells are lost.22
We also assessed whether or not the amount of laser energy used correlated with corneal change and found no such association at any time point. There was, however, a weak but significant correlation between a higher total laser energy and a greater CCT at 1 month post SLT, although this association was not significant at 1 week. This may signify that higher laser energy results in a more pronounced stromal remodeling and corneal healing contributing to a greater likelihood of subsequent return to baseline CCT at 1 month.
We are uncertain of the factors that may have contributed to the improvement of mean BCVA from 0.3 LogMAR at baseline to 0.2 LogMAR at 1 month post SLT. Given that visual testing results may fluctuate between visits, one cannot rule out the possibility of a placebo effect accounting for the favorable shift in BCVA, perhaps as a result of subjects mistakenly associating SLT as a laser refractive procedure.
The 11 control eyes (10% of the subject population) were included mainly for the calculation of repeatability of the three corneal investigations. Although the control sample was sufficient to demonstrate high reproducibility of the corneal investigations (average ICC of 0.997), the sample size was insufficient to detect any significant differences between the treated vs untreated fellow eyes. Future studies using the fellow untreated eye as controls would be ideal but many of the subjects in this study had bilateral disease and opted for bilateral treatment in the same setting.
To the best of our knowledge, this is the largest series documenting the transient changes in corneal endothelial cell count and CCT after a single session of SLT. It is possible that the immediate reduction in IOP immediately post SLT may be attributed by a falsely low reading from subclinical corneal edema. Although this study demonstrated the reversibility of corneal parameters in the early post SLT period, further follow-up studies are needed to establish more long-term effects and patients undergoing SLT should be aware of the risk of potential corneal changes.

References
Ramulu PY, Corcoran KJ, Corcoran SL, Robin AL . Utilization of various glaucoma surgeries and procedures in Medicare beneficiaries from 1995 to 2004. Ophthalmology 2007; 114: 2265–2270.
Latina MA, De Leon JM . Selective laser trabeculoplasty. Ophthalmol Clin North Am 2005; 18 (3): 409–419 vi.
Latina MA, Sibayan SA, Shin DH, Noecker RJ, Marcellino G . Q-switched 532-nm Nd:YAG laser trabeculoplasty (selective laser trabeculoplasty): a multicenter, pilot, clinical study. Ophthalmology 1998; 105 (11): 2082–2088 discussion 2089–2090.
Latina MA, Tμmbocon JA . Selective laser trabeculoplasty: a new treatment option for open angle glaucoma. Curr Opin Ophthalmol 2002; 13 (2): 94–96.
Melamed S, Ben Simon GJ, Levkovitch-Verbin H . Selective laser trabeculoplasty as primary treatment for open-angle glaucoma: a prospective, nonrandomized pilot study. Arch Ophthalmol 2003; 121 (7): 957–960.
Mahdy MA . Efficacy and safety of selective laser trabeculoplasty as a primary procedure for controlling intraocular pressure in primary open angle glaucoma and ocular hypertensive patients. Sultan Qaboos Univ Med J 2008; 8 (1): 53–58.
McIlraith I, Strasfeld M, Colev G, Hutnik CM . Selective laser trabeculoplasty as initial and adjunctive treatment for open-angle glaucoma. J Glaucoma 2006; 15 (2): 124–130.
Shazly TA, Smith J, Latina MA . Long-term safety and efficacy of selective laser trabeculoplasty as primary therapy for the treatment of pseudoexfoliation glaucoma compared with primary open-angle glaucoma. Clin Ophthalmol 2010; 5: 5–10.
Regina M, Bunya VY, Orlin SE, Ansari H . Corneal edema and haze after selective laser trabeculoplasty. J Glaucoma 2011; 20 (5): 327–329.
Armstrong RA, Eperjesi F, Gilmartin B . The application of analysis of variance (ANOVA) to different experimental designs in optometry. Ophthalmic Physiol Opt 2002; 22: 1–9.
Juzych MS, Chopra V, Banitt MR, Hughes BA, Kim C, Goulas MT et al. Comparison of long-term outcomes of selective laser trabeculoplasty versus argon laser trabeculoplasty in open-angle glaucoma. Ophthalmology 2004; 111 (10): 1853–1859.
White AJ, Mukherjee A, Hanspal I, Sarkies NJ, Martin KR, Shah P . Acute transient corneal endothelial changes following selective laser trabeculoplasty. Clin Experiment Ophthalmol 2013; 41 (5): 435–441.
Brooks AM, Grant G, Gillies WE . Reversible corneal endothelial cell changes in diseases of the anterior segment. Aust N Z J Ophthalmol 1987; 15 (4): 283–289.
Nakakura S, Imamura H, Nakamura T . Selective laser trabeculoplasty for glaucoma after penetrating keratoplasty. Optom Vis Sci 2009; 86 (4): e404–e406.
Lai JS, Chua JK, Tham CC, Lam DS . Five-year follow up of selective laser trabeculoplasty in Chinese eyes. Clin Experiment Ophthalmol 2004; 32 (4): 368–372.
Ho CL, Lai JS, Aquino MV, Rojanapongpun P, Wong HT, Aquino MC et al. Selective laser trabeculoplasty for primary angle closure with persistently elevated intraocular pressure after iridotomy. J Glaucoma 2009; 18 (7): 563–566.
Katz LJ CME: Selective Laser Trabeculoplasty for Glaucoma Therapy. Review of Ophthalmology; 2003 http://www.revophth.com/content/d/features/i/1342/c/25695/. (accessed 8 October 2013).
Huang Y, Zhang M, Huang C, Chen B, Lam DS, Zhang S et al. Determinants of postoperative corneal edema and impact on goldmann intraocular pressure. Cornea 2011; 30 (9): 962–967.
Hayes S, Boote C, Tuft SJ, Quantock AJ, Meek KM . A study of corneal thickness, shape and collagen organisation in keratoconus using videokeratography and X-ray scattering techniques. Exp Eye Res 2007; 84 (3): 423–434.
Meek KM, Leonard DW, Connon CJ, Dennis S, Khan S . Transparency swelling and scarring in the corneal stroma. Eye (Lond) 2003; 17 (8): 927–936.
Tanaka T, Furutani-Miura S, Nakamura M, Nishida T . Immunohistochemical study of localization of extracellular matrix after holmiμm YAG laser irradiation in rat cornea. Jpn J Ophthalmol 2000; 44 (5): 482–488.
Kotecha A . What biomechanical properties of the cornea are relevant for the clinician? Surv Ophthalmol 2007; 52 (Suppl 2): S109–S114.
Acknowledgements
We thank Ms Catherine Chan and Mr Keith Leung.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lee, J., Chan, J., Chang, R. et al. Corneal changes after a single session of selective laser trabeculoplasty for open-angle glaucoma. Eye 28, 47–52 (2014). https://doi.org/10.1038/eye.2013.231
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2013.231
Keywords
This article is cited by
-
Selective laser trabeculoplasty is safe and effective in patients previously treated with prostaglandin analogs: An evidence-based review
International Ophthalmology (2022)
-
Comparison of the effects of 180° and 360° applications of selective laser trabeculoplasty on intraocular pressure and cornea
International Ophthalmology (2020)
-
Corneal topography and angle parameters after laser iridotomy combined with iridoplasty assessed by dual Scheimpflug analyzer
International Ophthalmology (2020)
-
Could adverse effects and complications of selective laser trabeculoplasty be decreased by low-power laser therapy?
International Ophthalmology (2019)
-
Corneal endothelial changes following a single session of selective laser trabeculoplasty for pseudoexfoliative glaucoma
International Ophthalmology (2018)