Abstract
Purpose
To describe the clinical features and outcomes among eyes with choroidal neovascularization (CNV) in children and adolescents.
Methods
A total of 36 eyes of 27 patients <18 years of age diagnosed with CNV between January 1978 and December 2008 were retrospectively reviewed. CNV was clinically diagnosed in all patients and its presence was confirmed by fundus fluorescein angiography (FFA). A total of 19 eyes underwent treatment. Anatomical outcome was evaluated as regressed/persistent/recurrent CNV. Snellen’s values for best corrected visual acuity (BCVA) were converted to logMAR for statistical calculations.
Results
Of the 27 patients, 17 (63%) were male. Nine (33.3%) of the 27 patients had bilateral CNV. At presentation, CNV was active in 22 (61.1%) eyes and regressed in 14 (28.9%) eyes. All active CNV cases were ‘classic’ type, with the majority (80.5%) being subfoveal. The mean greatest linear dimension (GLD) was 3.16±1.94 mm (range, 0.9–10.15). The most common cause (41.7%) was post-inflammatory. The mean duration to regression in treated eyes was 103.53 days (15 eyes). Recurrence was noted in three (8.3%) eyes. The mean duration to first recurrence was 260 days (range, 90–390), and the mean follow-up duration was 779.53±988.00 days.
Conclusion
CNV remains a cause of significant visual decline in children and adolescents. Male predominance, post-inflammatory etiology, bilateral affection, and subfoveal location are noteworthy, with a high regression rate in response to treatment. Re-treatment is required in a limited number of cases.
Similar content being viewed by others
Introduction
Choroidal neovascularization (CNV) is a significant cause of central visual loss in children1 and adults, visual loss being particularly overwhelming in cases with subfoveal neovascularization.2 Although the prevalence of blindness is lower in children than in adults,3 children bear a greater burden of blindness because of much higher disability-adjusted life years (DALY).4 Moreover, a blind child faces considerable challenges in education and emotional development.5
There are some obvious differences in CNV between children and adults: first, rarity of macular degeneration and myopic fundus changes6 (the two most common causes of adult-onset CNV) at young age; second, lack of calcification and thickening of Bruch’s membrane (which is otherwise frequently observed among adults);7 and third, presence of solitary subretinal in-growth sites unlike adult cases in which multiple in-growth sites are common.8 All these factors may make the natural course, prognosis, and treatment outcomes more favorable among young subjects.9 Further, several etiological aspects related to CNV in children and adolescents remain imprecise. Although causes of CNV in young patients have been reviewed before,10, 11, 12 there are no large studies comparing the relative frequency of the causes in subjects below 18 years of age. Existing anecdotal reports do suggest a favorable natural course with spontaneous involution in about 58% of cases.1, 13 In the present study, we describe the causes, clinical profiles, and anatomical and visual outcomes with different treatment modalities among eyes with CNV in children and adolescents.
Materials and methods
This retrospective study was conducted at a tertiary eye care center. The study and data collection conformed to all local laws and were compliant with the principles of the Declaration of Helsinki. As per policy, the institutional review board’s approval was waived, as this was a retrospective study. Children and adolescents <18 years of age presenting between January 1978 and December 2008 and diagnosed with CNV were included. All subjects were Asian–Indians. Best corrected visual acuity (BCVA) was recorded with Snellen’s chart, and cycloplegic refraction was performed. Snellen’s values for BCVA were converted to logMAR values for statistical calculations. CNV was diagnosed clinically and confirmed using fundus fluorescein angiography (FFA). However, the ability to undergo FFA was not essentially an inclusion criterion. The CNV location was defined according to MPS protocol14 as subfoveal, juxtafoveal, and extrafoveal, and as peripapillary/juxtapapillary for CNV adjacent to the optic disc. Greatest linear dimension (GLD) was recorded in 31 of the 36 eyes. In the remaining five eyes, GLD could not be measured because of some photographs being captured on negative photographic films early on in the study period. Optical coherence tomography (OCT) was performed using Spectral Domain OCT (SD OCT) (3D OCT-1000, Topcon Inc., Tokyo, Japan) in the subjects who presented during the later part of the study.
Results
A total of 36 eyes from 27 patients were included. The left eye was affected in 19 patients (70.3%), whereas the right eye was affected in 17 (62.9%) of the 27 patients. Bilateral involvement was noted in nine (33.3%) patients. Of the 36 eyes with ‘clinically active’ CNV at presentation, 22 (61.1%) were of ‘classic’ type on FFA.
Table 1 depicts the demographic features of the 36 eyes of 27 patients. The mean age at presentation was 12.7±3.0 years (range, 7–17). Seventeen of the 27 (63%) patients were male. Twenty-nine (80.5%) of the 36 eyes had subfoveal CNV. The mean GLD was 3.16±1.94 mm (range, 0.9–10.15). The mean follow-up duration was 25.98±32.93 months. The two most common etiologies were post-inflammatory (41.7%) and Best’s disease (25.0%). Seven (19.4%) eyes of five patients had CNV associated with unclassified choroiditis (Eyes 1–7), their mean age being 13.6±2.5 years. Three (8.3%) eyes of two patients had CNV associated with serpiginous choroiditis (Eyes 8–10), their mean age being 10.0±5.2 years. Two (5.5%) eyes of a single patient (17 years old) had CNV associated with Vogt–Koyanagi–Harada disease (VKH) (Eyes 11, 12). Two (5.5%) eyes of two patients had CNV associated with toxoplasmosis (Eyes 13, 14), with the mean age being 13.0±1.4 years. One (2.8%) eye of one patient (14 years old) had CNV associated with presumed ocular histoplasmosis syndrome (POHS) (Eye 15). Both eyes (Eyes 29 and 30) of an 11-year-old patient were diagnosed with CNV associated with viral retinitis, with coexisting ONH drusen. Nine (25.0%) eyes of five patients had CNV associated with Best’s disease (Eyes 16–24), the mean age being 11.1±3.0 years. Four (11.1%) eyes had idiopathic CNV (Eyes 25–28), the mean age being 13.75±2.5 years. Three (8.3%) eyes had CNV associated with ONH drusen (Eyes 29–31); the mean age was 11.0 years. Two (5.5%) eyes had CNV associated with Stargardt’s disease (Eyes 32 and 33); the mean age was 12.5±2.1 years. Two (5.5%) eyes of two patients had CNV associated with myopia (Eyes 34 and 35), one of which (Eye 34) had coexisting osteogenesis imperfecta. The mean age of patients was 14.5±3.5 years. We encountered one case (2.8%) of traumatic CNV (eye 36) in the absence of obvious choroidal rupture, associated with blunt trauma sustained 6 years ago.
Of the 22 eyes with active CNV, 18 received treatment at our institute and four did not undergo any treatment because of poor visual potential or treatment being refused by the parents of the patient. Of the 18 treated eyes, 16 showed regression of CNV, with the mean duration to treatment-induced regression being 3.45 months. Of four untreated eyes, two (eyes 6 and 33) developed spontaneous regression of CNV, with the mean duration to spontaneous regression being 1.5 months (range, 1–2). Of 14 eyes with involuted CNV at presentation (Supplementary Table 1), 13 had never been treated (had spontaneously regressed CNV) and one (eye 11) had received focal laser photocoagulation for CNV (elsewhere) shortly before presenting to us. Thus, in 17 eyes (the Natural history group/Group 1), we studied the natural course of CNV. The remaining 19 eyes were treated in our study (Treatment group/Group 2). Of the 17 untreated eyes of Group 1, 15 had spontaneous regression, and two had persistent CNV until the last follow-up (Eyes 22 and 24). None of the eyes in group 1 developed recurrence. Of the two eyes in group 1 that had longer follow-up (>6 months), BCVA was maintained in one (Eye 8) and improved in other (Eye 17) at last visit. Of the 19 treated eyes of group 2, regression of CNV was noted in 17 eyes and persistence in two eyes (Eyes 29 and 30). Of the 17 eyes showing regression, three developed recurrence of CNV. All three eyes with recurrent CNV were re-treated and CNV was regressed at last follow-up.
Table 2 shows treatment details of 19 eyes of Group 2 in accordance with changing treatment trends for CNV over time. Of eight eyes with persistent CNV, six were re-treated and required an average 1.4 re-treatments for (FFA-documented) regression of CNV. Recurrence was noted in three eyes, two with inflammatory CNV (Eyes 9, 10) and one with myopic CNV (Eye 34). The mean time to first recurrence in 3 eyes was 6.67 months (range, 2–9) from known regression and 8.67 months (range, 3–13) from last treatment. The longest recurrence-free follow-up was 113.37 months for inflammatory CNV and 19.33 months for myopic CNV. Thus, at the final visit, 17 of 19 (89.4%) eyes had documented regression of CNV, whereas two eyes (Eyes 29 and 30) had persistent CNV, albeit with a short follow-up.
Discussion
Clinical and demographic profile
There was slight male preponderance. No gender predilection exists in the published literature. In our study, the youngest patient was 7 years old and the one reported in the literature is 4 months of age.15
Etiology
The two most common etiologies were post-inflammatory and Best’s disease.
Inflammatory CNV
Inflammatory CNV is the most common type of CNV among children and adolescents (Figure 1).12, 16
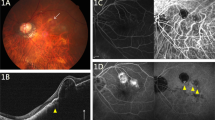
Bilateral CNV in a 15-year-old boy (Eyes 3 and 4) due to post-inflammatory cause. At presentation, color fundus photographs of the right (a) and left (b) eye shows a yellowish, subretinal lesion extending from the disc to the fovea, suggestive of CNV with ‘juxtapapillary’ and ‘subfoveal’ components. CNV in the right eye appears regressed, whereas the left eye shows fresh subretinal hemorrhage and subretinal fluid. (c and d) FFA of the left eye showing early hyperfluorescence (arteriovenous phase, c) and intense late leakage (d) corresponding to an active CNV membrane. (e) FFA of the right eye reveals hyperfluorescence with late staining, suggestive of regressed CNV. (f) Ten weeks after surgical removal of CNV, a color fundus photograph of the left eye reveals atrophic scar tissue and pigmentation due to reactive hyperplasia of retinal pigment epithelium. (g and h) FFA of the left eye reveals areas of speckled, transmitted fluorescence, suggestive of pigment epithelial atrophy and blocked fluorescence due to reactive hyperplasia of pigment epithelium (g). Late phase reveals staining without leakage (h). Absence of CNV is noteworthy.
Unclassified choroiditis Unclassified choroiditis was the most common etiology. These were the eyes in which signs of inflammation including active/scarred chorioretinitis/retinal vasculitis were present but did not fit into a definitive disease entity.17
Serpiginous choroiditis Serpiginous-like choroidopathy is seen in 30–60-year-olds. However, it has been reported to occur at younger ages of <8 years in Caucasian18 and Indian populations.19 CNV with serpiginous choroidopathy has poor visual prognosis.20
Vogt–Koyanagi–Harada disease This syndrome typically affects adults 20–50 years of age; however, a few cases in young children affected with CNV have been reported.21, 22 In children with VKH, the incidence of CNV is estimated to be as high as 70%.23, 24, 25 CNV in VKH is known to have a poor visual prognosis.26
Toxoplasmosis Ocular toxoplasmosis is a common cause of inflammatory CNV in children and adolescents.15, 17, 27, 28, 29, 30
Presumed ocular histoplasmosis syndrome POHS has been reported to be the most common cause of inflammatory CNV in children and adolescents.12, 13, 17 POHS shows a geographical predilection and, conventionally, has been noted to occur exclusively in the United States, with no significant numbers reported in any of the other regions.31 POHS has rarely been reported in India, including a recent series of three cases with CNV.32, 33
Viral retinopathy Viral retinopathy has been reported to cause CNV formation in children.34, 35 Rubella retinopathy is asymptomatic, unless it is made complicated by the development of CNV at the macula.34, 35
Best’s disease
Being the second common cause described in our study, Best’s disease is a known cause of CNV in children and adolescents.1, 36, 37, 38, 39, 40
Other etiologies
Idiopathic CNV, another common cause seen in our study, has been described frequently.10, 13, 16, 17, 40, 41, 42 The reported prevalence of ONH drusen in children is 0.4%.43 Although CNV associated with ONH drusen is typically peripapillary, subfoveal extension can occur.44, 45 High myopia can induce CNV, commonly in older patients between 30 and 50 years of age, being rare in children.46 Kobayashi et al6 reported the absence of CNV development over a 10-year follow-up in 80 eyes with high myopia in children aged ≤8 years. This may be because ageing, in addition to mechanical stretching of the eyeball, might be important for the development of predisposing factors such as RPE atrophy and lacquer cracks.47 However, myopic CNV has been reported in children.48, 49, 50 In our study, of the two patients with myopic CNV, one had coexisting osteogenesis imperfecta.51
In subjects sustaining trauma, CNV has been reported in the presence of choroidal ruptures during the healing phase.52, 53, 54, 55, 56 However, in the present study, we had one case of traumatic CNV in the absence of choroidal rupture. Other causes of CNV in children and adolescents are retinal dystrophies, including Stargardt disease,57 choroideremia,58 North Carolina macular dystrophy,59 and other macular dystrophies.60
Morphological characteristics of CNV
CNV in children and adolescents are known to be type 2 membranes, having features of ‘classic’ CNV on FFA.13 In this series, all membranes that were active (22 eyes) at presentation were of ‘classic’ type. It is known that children lack thickening and calcification of Bruch’s membrane and diffuse disruption of RPE, which are seen in older patients with AMD.7 CNV in children is more likely to have a solitary in-growth site, whereas the majority of CNV cases in patients with AMD have multiple in-growth sites.8 These are the reasons for a high rate of spontaneous regression of CNV in children and for making surgical excision technically complete and recurrences less likely. Components of CNVMs in children are similar to those of adults, except for the absence of basal laminar deposits.17
Location of CNV
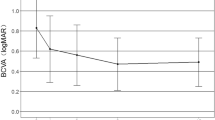
The most common location of CNV was subfoveal, which was consistent with other reports.1, 16 Among 29 eyes with a subfoveal component, the mean logMAR BCVA was 1.21 for eyes that had regressed CNV at presentation and 1.08 for eyes that had active CNV at presentation. Eyes with active CNV that regressed after treatment showed improvement in mean BCVA to 0.82, suggesting the favorable impact of treatment for active CNV in children and adolescents (Supplementary Table 2).
One eye (Eye 31) had regressed extrafoveal CNV at presentation and was observed. The mean BCVA of this eye was 0.6. Peripapillary CNV in children and adolescents has been reported to occur as a primary membrane61 in POHS,16 optic disc drusen,62, 63, 64, 65 chronic papilledema,66 pseudopapilledema,67 idiopathic intracranial hypertension,68 malignant hypertension,69 idiopathic anomalies,70, 71 and optic nerve head cavitary anomalies.72
Management
For the sake of better understanding, we divided the patients in our series into two groups on the basis of whether or not they underwent any form of treatment.
Natural history group/Group 1
Of the 17 eyes in group 1, 15 developed spontaneous regression of CNV. Spontaneous regression has previously also been reported to be very common in pediatric CNV.1, 13 Hence, observation of CNVs in children may appear to be a reasonable approach; however, it is difficult to predict which CNVs will regress and which will persist/progress to result in permanent vision loss without any treatment. Furthermore, visual outcome in eyes with successfully treated subfoveal CNV was noted to be better than in eyes with spontaneously regressed subfoveal CNV in the present study.
Treatment group/Group 2
A total of 19 eyes were treated in our study. With an evolution in the management of such eyes, some of these treatment modalities are now important only from a historic point of view.
Historic treatments
Before the anti-vascular endothelial growth factor (VEGF) era, treatment options for CNV in children and adolescents were limited to laser photocoagulation for extrafoveal73 and juxtafoveal CNV, and photodynamic therapy (PDT) for subfoveal CNV (Supplementary Table 3).30, 74, 75 For subfoveal CNV, alternative treatment included surgical removal of CNV16, 40, 45 and transpupillary thermotherapy (TTT). For peripapillary CNV in children, good results of surgery,64 laser photocoagulation,70 and PDT have been reported.71
PDT with verteporfin can be considered in the pediatric population; several case reports suggest that pediatric patients require fewer re-treatments compared with adult patients to stabilize CNV and achieve an improvement in visual acuity; however, atrophic changes in RPE can occur.36, 65, 74, 75 In our study, both eyes undergoing surgical removal of CNV (100%) showed improved visual outcome without recurrence. In eyes undergoing surgical removal of subfoveal CNV, Sears et al17 reported visual improvement in 83.3% eyes and a recurrence rate of 33%; Uemura and Thomas16 reported visual improvement in 72% and a recurrence rate of 35%. The better results in terms of visual improvement and recurrence after surgical removal of CNV in our study could be because of the fewer number of patients as compared with the other two studies. Moreover, both cases in our study had a shorter mean follow-up (average 3 months) and one eye had juxtapapillary CNV.
Current treatment options
The use of newer anti-VEGF agents in pediatric CNV has been reported recently for both bevacizumab37, 76, 77 and ranibizumab.44 Isolated reports are available for pegaptanib sodium.78 Combination treatments have also been reported for CNV in children and adolescents.29, 49 In our series, one eye was treated with intravitreal bevacizumab injection and another with a combination of PDT and bevacizumab. With the increasing use of anti-VEGF agents in younger patients for CNV, safety and long-term visual outcomes remain a concern. VEGF has an important role in normal angiogenesis, regulation of vessel permeability, and in maintenance of the blood–brain barrier. Hence, the long-term results of inhibiting these functions by using anti-VEGF agents in children need to be further evaluated before concluding that these agents are safe in children. In our case series, we did not observe any short-term adverse ocular or systemic side effects of treatment with intravitreal anti-VEGF agents. Moreover, no adverse events have been reported with the use of intravitreal anti-VEGF agents in the published literature for the treatment of pediatric CNV and other pediatric eye diseases.37, 44, 76, 77 Fewer injections of anti-VEGF agents seem to be required to stabilize CNVs in children compared with adults.76 The reason might be the better health of the RPE pump in younger subjects than in adults. Fewer injections may potentially decrease the risk of adverse effects of anti-VEGF agents in younger patients.76 In children, as reported by Avery et al,79 the use of ranibizumab instead of bevacizumab may lower systemic exposure, given its much shorter serum half-life and as found in several animal studies.
In the era of treatment with anti-VEGF agents, the ability of OCT to provide detailed information in a noninvasive manner is of great importance. The noninvasive OCT is especially useful over invasive FFA, as repeated FFA may not be possible in children. Furthermore, the CNV in children is classic. Classic CNVs, being localized above the retinal pigment epithelium, are clearly visualized by SD OCT. SD OCT allows to detect in detail the structural changes in the retinal pigment epithelium–photoreceptor complex and to image the architecture of the CNV during the course of treatment.80 In classic CNV, indocyanine green angiography and FFA seem to underestimate the extension of the neovascular complex and the associated retinal pathologic features compared with SD OCT imaging.81 Sulzbacher et al81 reported that as SD OCT was more reliable in detecting leakage, re-treatment based on SD OCT parameters should be more effective and could replace angiographic imaging, particularly because pharmacologic treatment works by reducing leakage rather than by showing a true antiproliferative effect.
Treatment of CNV due to inflammatory causes
There are reports of successful regressions of CNV in children with inflammation control alone with systemic corticosteroids with or without immunosuppressants.82 However, this may not always be successful, necessitating additional treatment such as PDT.21 Reports on cases of inflammatory CNV in patients younger than 18 years treated with PDT have suggested the possibility of improved outcomes.21, 22 Almony et al83 reported the results of surgery in six patients with peripapillary CNV secondary to POHS who were ≤18 of age.
Recent interest has focused on the antiangiogenic approach for the treatment of inflammatory CNV.77 Intravitreal bevacizumab has been used for CNV related to inflammatory diseases in patients younger than 18 years when underlying inflammation is controlled.77 Combination treatment has also been reported for inflammatory CNV.29
Outcomes
Natural history group/Group 1
Spontaneous regression Spontaneous regression of CNV was seen in 15 (41.7%) eyes. Of these, six eyes had a follow-up of more than 1 month; visual improvement was seen in one eye and stabilization in five eyes. A previous report showed spontaneous involution in 58% of subretinal neovascular membranes in children and adolescents,1 with 81.8% of these achieving a final visual acuity of 20/50 or better. Literature reports that the natural course of CNV seems to be more favorable in pediatric patients than in adults.1, 17
Persistence in natural course Two eyes had active CNV that was not treated because of unwillingness of the patient, and documented regression of CNV was not available because of lack of follow-up.
Treatment group/Group 2
Regression with treatment Mean BCVA (logMAR 1.21) in eyes with successfully treated subfoveal CNV was better than in eyes with spontaneously regressed subfoveal CNV (logMAR 0.632), as shown in the Supplementary Tables. This highlights the importance of early diagnosis and treatment, despite the high possibility of spontaneous regression. Considering the time to regression by etiology, post-inflammatory CNV (due to serpiginous choroiditis and POHS), CNV secondary to Best’s disease, and myopic CNV took longer to regress (>4 months), whereas unclassified inflammatory, VKH, toxoplasmosis, Stargardt disease, and idiopathic CNV regressed earlier (<3 months). The longest time to regression after treatment was noted for myopic CNV (Eye 35), as shown in Table 2. Although another eye with myopic CNV (Eye 34) showed signs of scarring after 1 month of treatment, it reactivated twice at 3 and 16 months after the first treatment. This emphasizes the recalcitrant nature of myopic CNV in children and adolescents and the need for prolonged monitoring.
Persistence Among the 19 treated eyes, two eyes did not show regression of CNV with primary treatment and these patients were advised surgical management. Patients denied treatment and there was no further follow-up. Subfoveal CNV cases that showed no signs of regression in children and adolescents were reported to be associated with severe visual loss (<20/200) secondary to disciform scar formation.1 A longer follow-up of such patients in our series could have helped understand this aspect better.
Recurrence The overall recurrence rate in our study was 8.3% (three eyes). This is probably lower than the true incidence because of short follow-up. Interestingly, all three recurrences were noted in treated eyes but none in eyes with spontaneous regression.
This study has some inherent limitations, the major one being the lack of long-term follow-up. Another limitation is the perceived lack of OCT correlation in several cases. However, some patients were treated before the advent of OCT. Nevertheless, in the current scenario, OCT is a standard investigation and helps generate useful information regarding treatment planning, efficacy, and follow-up. Even though conventional FFA was performed in all eyes, wide-field retinal imaging and angiography (for example, RetCam, Clarity Medical Systems Inc., Pleasanton, CA, USA) are other useful options. Furthermore, as the present study comprises patients treated many years ago with historic treatment modalities, it would be fair to say that management strategies are still evolving.
CNV remains a cause of significant visual decline in children and adolescents.84 Male predominance, post-inflammatory etiology, bilateral affection, and subfoveal location are noteworthy features. Regression rates are high in response to treatment. However, re-treatment is required in a limited number of cases.

References
Goshorn EB, Hoover DL, Eller AW, Friberg TR, Jarrett WH 2nd, Sorr EM . Subretinal neovascularization in children and adolescents. J Pediatr Ophthalmol Strabismus 1995; 32: 178–182.
Fine SL, Wood WJ, Isernhagen RD, Singerman LJ, Bressler NM, Folk JC et al. Laser treatment for subfoveal neovascular membranes in ocular histoplasmosis syndrome: results of a pilot randomized clinical trial. Arch Ophthalmol 1993; 111: 19–20.
Gilbert C, Foster A, Negrel AD, Thylefor B . Childhood blindness: a new form for recording causes of visual loss in children. Bull World Health Organ 1993; 71: 485–489.
World Health Organization. Report of WHO/IAPB Scientific Meeting, Childhood Blindness Prevention. WHO/PBL/87: London, 2001.
Cass HD, Sonsen PM, McConachie HR . Developmental setback in severe visual impairment. Arch Dis Child 1994; 70: 192–196.
Kobayashi K, Ohno-Matsui K, Kojima A, Shimada N, Yasuzumi K, Yoshida T et al. Fundus characteristics of high myopia in children. Jpn J Ophthalmol 2005; 49: 306–311.
Spraul CW, Grossniklaus HE . Characteristics of Drusen and Bruch's membrane in postmortem eyes with age-related macular degeneration. Arch Ophthalmol 1997; 115: 267–273.
Melberg NS, Thomas MA, Burgess DB . The surgical removal of subfoveal choroidal neovascularization. Ingrowth site as a predictor of visual outcome. Retina 1996; 16: 190–195.
Gass JD . Biomicroscopic and histopathologic considerations regarding the feasibility of surgical excision of subfoveal neovascular membranes. Am J Ophthalmol 1994; 118: 285–298.
Spaide RF . Choroidal neovascularization in younger patients. Curr Opin Ophthalmol 1999; 10: 177–181.
Cohen SY, Laroche A, Leguen Y, Soubrane G, Coscas GJ . Etiology of choroidal neovascularization in young patients. Ophthalmology 1996; 103: 1241–1244.
Sivaprasad S, Moore AT . Choroidal neovascularisation in children. Br J Ophthalmol 2008; 92: 451–454.
Wilson ME, Mazur DO . Choroidal neovascularization in children: report of five cases and literature review. J Pediatr Ophthalmol Strabismus 1988; 25: 23–29.
Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Macular Photocoagulation Study Group. Arch Ophthalmol 1991; 109: 1220–1231.
Mavrikakis E, Levin AV, Lam WC . Choroidal neovascularization secondary to congenital toxoplasmosis in an infant. Can J Ophthalmol 2010; 45: e11–e12.
Uemura A, Thomas MA . Visual outcome after surgical removal of choroidal neovascularization in pediatric patients. Arch Ophthalmol 2000; 118: 1373–1378.
Sears J, Capone A Jr, Aaberg T Sr, Lewis H, Grossniklaus H, Sternberg P Jr et al. Surgical management of subfoveal neovascularization in children. Ophthalmology 1999; 106: 920–924.
Christmas NJ, Oh KT, Oh DM, Folk JC . Long-term follow-up of patients with serpinginous choroiditis. Retina 2002; 22: 550–556.
Gupta V, Agarwal A, Gupta A, Bambery P, Narang S . Clinical characteristics of serpiginous choroidopathy in North India. Am J Ophthalmol 2002; 134: 47–56.
Erkkila H, Laatikainen L . A follow up study of serpiginous choroiditis. Acta Ophthalmol 1981; 59: 707–718.
Farah ME, Costa RA, Muccioli C, Guia TA, Belfort R Jr . Photodynamic therapy with verteporfin for subfoveal choroidal neovascularization in Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol 2002; 134: 137–139.
Nowilaty SR, Bouhaimed M, Photodynamic Therapy Study Group. Photodynamic therapy for subfoveal choroidal neovascularisation in Vogt-Koyanagi-Harada disease. Br J Ophthalmol 2006; 90: 982–986.
Abu El-Asrar AM, Al-Kharashi AS, Aldibhi H, Al-Fraykh H, Kangave D . Vogt-Koyanagi-Harada disease in children. Eye (Lond) 2008; 22: 1124–1131.
Tabbara KF, Chavis PS, Freeman WR . Vogt-Koyanagi-Harada syndrome in children compared to adults. Acta Ophthalmol Scand 1998; 76: 723–726.
Soheilian M, Aletaha M, Yazdani S, Dehghan MH, Peyman GA . Management of pediatric Vogt-Koyanagi-Harada (VKH)-associated panuveitis. Ocul Immunol Inflamm 2006; 14: 91–98.
Rubsamen PE, Gass JD . Vogt-Koyanagi-Harada syndrome. Clinical course, therapy and long term visual outcome. Arch Ophthalmol 1991; 109: 682–687.
Mauget-Faysse M, Mimoun G, Ruiz-Moreno JM, Quaranta-El Maftouhi M, De Laey JJ, Postelmans L et al. Verteporfin photodynamic therapy for choroidal neovascularization associated with toxoplasmic retinochoroiditis. Retina 2006; 26: 396–403.
Benevento JD, Jager RD, Noble AG, Latkany P, Mieler WF, Sautter M et alToxoplasmosis Study Group. Toxoplasmosis-associated neovascular lesions treated successfully with ranibizumab and antiparasitic therapy. Arch Ophthalmol 2008; 126: 1152–1156.
Rishi P, Venkataraman A, Rishi E . Combination photodynamic therapy and bevacizumab for choroidal neovascularization associated with toxoplasmosis. Indian J Ophthalmol 2011; 59: 62–64.
Giansanti F, Virgili G, Varano M, Tedeschi M, Rapizzi E, Giacomelli G et al. Photodynamic therapy for choroidal neovascularization in pediatric patients. Retina 2005; 25: 590–596.
Chang JH, Wakefield D . Uveitis: a global perspective. Ocul Immunol Inflamm 2002; 10: 263–279 (Review).
Goswami RP, Pramanik N, Banerjee D, Raza MM, Guha SK, Maiti PK . Histoplasmosis in eastern India: the tip of the iceberg? Trans R Soc Trop Med Hyg 1999; 93: 540–542.
Sinha R, Raju S, Garg SP, Venkatesh P, Talwar D . Presumed ocular histoplasmosis syndrome in India. Immunol Inflamm 2007; 15: 315–317.
Hirano K, Tanikawa A, Miyake Y . Neovascular maculopathy associated with rubella retinopathy. Jpn J Ophthalmol 2000; 44: 697.
Veloso CE, Costa RA, Oréfice JL, Oréfice F . Spontaneous involution of choroidal neovascularization secondary to rubella retinopathy. Eye (Lond) 2007; 21: 1429–1430.
Viola F, Villani E, Mapelli C, Staurenghi G, Ratiglia R . Bilateral juvenile choroidal neovascularization associated with Best's vitelliform dystrophy: observation versus photodynamic therapy. J Pediatr Ophthalmol Strabismus 2010; 47: 121–122.
Leu J, Schrage NF, Degenring RF . Choroidal neovascularisation secondary to Best's disease in a 13-year-old boy treated by intravitreal bevacizumab. Graefes Arch Clin Exp Ophthalmol 2007; 245: 1723–1725.
Mandal S, Sinha S, Venkatesh P, Vashisht N . Intravitreal bevacizumab in choroidal neovascularization associated with Best's vitelliform dystrophy. Indian J Ophthalmol 2011; 59: 262–263.
Rich R, Vanderveldt S, Berrocal AM, Mavrofrides EC, Murray TG, Gregori N . Treatment of choroidal neovascularization associated with Best's disease in children. J Pediatr Ophthalmol Strabismus 2009; 46: 306–311.
Jain K, Shafiq AE, Devenyi RG . Surgical outcome for removal of subfoveal choroidal neovascular membranes in children. Retina 2002; 22: 412–417.
Daniels AB, Jakobiec FA, Westerfeld CB, Hagiwara A, Michaud N, Mukai S . Idiopathic subfoveal choroidal neovascular membrane in a 21-month-old child: ultrastructural features and implication for membranogenesis. J AAPOS 2010; 14: 244–250.
Carneiro AM, Silva RM, Veludo MJ, Barbosa A, Ruiz-Moreno JM, Falcão MS et al. Ranibizumab treatment for choroidal neovascularization from causes other than age-related macular degeneration and pathological myopia. Ophthalmologica 2011; 225: 81–88.
Erkkilä H . Clinical appearance of optic disc drusen in childhood. Albrecht Von Graefes Arch Klin Exp Ophthalmol 1975; 193: 1–18.
Gregory-Evans K, Rai P, Patterson J . Successful treatment of subretinal neovascularization with intravitreal ranibizumab in a child with optic nerve head drusen. J Pediatr Ophthalmol Strabismus 2009 e-pub 21 August 2009; doi:10.3928/01913913-20090818-03.
Sullu Y, Yildiz L, Erkan D . Submacular surgery for choroidal neovascularization secondary to optic nerve drusen. Am J Ophthalmol 2003; 136: 367–370.
Grossniklaus HE, Green WR . Pathologic findings in pathologic myopia. Retina 1992; 12: 127–133.
Ohno-Matsui K, Yoshida T, Futagami S, Yasuzumi K, Shimada N, Kojima A et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol 2003; 87: 570–573.
Bottoni F, Tilanus M . The natural history of juxtafoveal and subfoveal choroidal neovascularization in high myopia. Int Ophthalmol 2001; 24: 249–255.
Potter MJ, Szabo SM, Ho T . Combined photodynamic therapy and intravitreal triamcinolone for the treatment of myopic choroidal neovascularization in a 13-year-old girl. Graefes Arch Clin Exp Ophthalmol 2006; 244: 639–641.
de Oliveira Dias JR, Rodrigues EB, Martinazzo M, Farah ME . Choroidal neovascularization in patient undergoing growth hormone treatment. Clin Ophthalmol 2009; 3: 89–90.
Rishi P, Rishi E, Venkatraman A . Intravitreal bevacizumab for treatment of choroidal neovascularization associated with osteogenesis imperfecta. Indian J Ophthalmol 2012; 60: 229–231.
Harissi-Dagher M, Sebag M, Gauthier D, Marcil G, Labelle P, Arbour JD . Photodynamic therapy in young patients with choroidal neovascularization following traumatic choroidal rupture. Am J Ophthalmol 2005; 139: 726–728.
Prasad A, Chirag CP, Puklin JE . Intravitreal bevacizumab in the treatment of choroidal neovascularization from a traumatic choroidal rupture in a 9-year-old child. Retinal Case Brief Rep 2009; 3: 125–127.
Piermarocchi S, Benetti E, Fracasso G . Intravitreal bevacizumab for posttraumatic choroidal neovascularization in a child. J AAPOS 2011; 15: 314–316.
Gross JG, King LP, de Juan E Jr, Powers T . Subfoveal neovascular membrane removal in patients with traumatic choroidal rupture. Ophthalmology 1996; 103: 579–585.
Abri A, Binder S, Pavelka M, Tittl M, Neumüller J . Choroidal neovascularization in a child with traumatic choroidal rupture: clinical and ultrastructural findings. Clin Experiment Ophthalmol 2006; 34: 460–463.
Klein R, Lewis RA, Meyers SM, Myers FL . Subretinal neovascularization associated with fundus flavimaculatus. Arch Ophthalmol 1978; 96: 2054–2057.
Endo K, Yuzawa M, Ohba N . Choroideremia associated with subretinal neovascular membrane. Acta Ophthalmol Scand 2000; 78: 483–486.
Rhee DY, Reichel E, Rogers A, Strominger M . Subfoveal choroidal neovascularization in a 3-year-old child with North Carolina macular dystrophy. J AAPOS 2007; 11: 614–615.
Mahajan VB, Russell SR, Stone EM . A new macular dystrophy with anomalous vascular development, pigment spots, cystic spaces, and neovascularization. Arch Ophthalmol 2009; 127: 1449–1457.
Lee EJ, Mavrikakis I, Fong K, Casswell AG . Primary peripapillary membrane in an 8-year-old boy. Eye (Lond) 2006; 20: 379–380.
Knape RM, Zavaleta EM, Clark CL 3rd, Khuddus N, Peden MC . Intravitreal bevacizumab treatment of bilateral peripapillary choroidal neovascularization from optic nerve head drusen. J AAPOS 2011; 15: 87–90.
Mehta P, Puri P, Talbot JF . Disc drusen and peripapillary subretinal neovascular membrane in a child with the VACTERL association. Eye (Lond) 2006; 20: 847–848.
Mateo C, Moreno JG, Lechuga M, Adán A, Corcóstegui B . Surgical removal of peripapillary choroidal neovascularization associated with optic nerve drusen. Retina 2004; 24: 739–745.
Silva R, Torrent T, Loureiro R, Travassos A, de Abreu JR . Bilateral CNV associated with optic nerve drusen treated with photodynamic therapy with verteporfin. Eur J Ophthalmol 2004; 14: 434–437.
Nguyen C, Borruat FX . Bilateral peripapillary subretinal neovessel membrane associated with chronic papilledema: report of two cases. Klin Monatsbl Augenheilkd 2005; 222: 275–278.
Anderson CJ, Zavel DW, Schlagel TF Jr, Meyer SM . Bilateral juxtapapillary subretinal neovascularization and pseudopapilledema in a three year old child. J Pediatr Ophthalmol Strabismus 1978; 15: 296–299.
Kaeser PF, Borruat FX . Peripapillary neovascular membrane: a rare cause of acute vision loss in pediatric idiopathic intracranial hypertension. J AAPOS 2011; 15: 83–86.
Browning AC, Mengher LS, Gregson RM, Amoaku WM . Visual outcome of malignant hypertension in young people. Arch Dis Child 2001; 85: 401–403.
Kiss S, Rizzo JF III, Mukai S . Peripapillary choroidal neovascularization in children. Invest Ophthalmol Vis Sci 2005; 46, E-Abstract 4084.
Yıldırım C, Cetin EN, Yayla K, Avunduk AM, Yaylalı V . Photodynamic therapy for unilateral idiopathic peripapillary choroidal neovascularization in a child. Int Ophthalmol 2011; 31: 333–335.
Yedavally S, Frank RN . Peripapillary subretinal neovascularization associated with coloboma of the optic nerve. Arch Ophthalmol 1993; 111: 552–553.
Shaikh S, Trese M . Infantile choroidal neovascularization associated with choroidal coloboma. Retina 2003; 23: 585–586.
Rishi P, Sharma T, Gopal L . Photodynamic therapy for childhood choroidal neovascular membrane associated with Best's vitelliform dystrophy. Retinal Cases Brief Rep 2009; 3: 288–292.
Mimouni KF, Bressler SB, Bressler NM . Photodynamic therapy with verteporfin for subfoveal choroidal neovascularization in children. Am J Ophthalmol 2003; 135: 900–902.
Kohly RP, Muni RH, Kertes PJ, Lam WC . Management of pediatric choroidal neovascular membranes with intravitreal anti-VEGF agents: a retrospective consecutive case series. Can J Ophthalmol 2011; 46: 46–50.
Kramer M, Axer-Siegel R, Jaouni T, Reich E, Hemo I, Priel E et al. Bevacizumab for choroidal neovascularization related to inflammatory diseases. Retina 2010; 30: 938–944.
Vinekar A, Sund N, Quiram P, Capone A Jr. . Choroidal neovascular membrane in persistent fetal vasculature syndrome managed with intravitreal pegaptanib sodium in an infant. Retina 2010; 30 (4 Suppl): S41–S44.
Avery RL . Extrapolating anti-vascular endothelial growth factor therapy into pediatric ophthalmology: promise and concern. J AAPOS 2009; 13: 329–331.
Coscas F, Querques G, Forte R, Terrada C, Coscas G, Souied EH . Combined fluorescein angiography and spectral-domain optical coherence tomography imaging of classic choroidal neovascularization secondary to age-related macular degeneration before and after intravitreal ranibizumab injections. Retina 2012; 32: 1069–1076.
Sulzbacher F, Kiss C, Munk M, Deak G, Sacu S, Schmidt-Erfurth U . Diagnostic evaluation of type 2 (classic) choroidal neovascularization: optical coherence tomography, indocyanine green angiography, and fluorescein angiography. Am J Ophthalmol 2011; 152: 799–806.e1.
Dees C, Arnold JJ, Forrester JV, Dick AD . Immunosuppressive treatment of choroidal neovascularization associated with endogenous posterior uveitis. Arch Ophthalmol 1998; 116: 1456–1461.
Almony A, Thomas MA, Atebara NH, Holekamp NM, Del Priore LV . Long-term follow-up of surgical removal of extensive peripapillary choroidal neovascularization in presumed ocular histoplasmosis syndrome. Ophthalmology 2008; 115: 540–545.e5.
Frank KE, Purnell EW . Subretinal neovascularization following rubella retinopathy. Am J Ophthalmol 1978; 86: 462–466.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Eye website
Supplementary information
Rights and permissions
About this article
Cite this article
Rishi, P., Gupta, A., Rishi, E. et al. Choroidal neovascularization in 36 eyes of children and adolescents. Eye 27, 1158–1168 (2013). https://doi.org/10.1038/eye.2013.155
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2013.155
Keywords
This article is cited by
-
Choroidal neovascularization in 111 eyes of children and adolescents
International Ophthalmology (2022)
-
Intravitreal anti-VEGF treatment for choroidal neovascularization secondary to traumatic choroidal rupture
BMC Ophthalmology (2019)
-
Intravitreal Ranibizumab for the Treatment of Visual Impairment Due to Choroidal Neovascularization Associated with Rare Diseases: Cost-Effectiveness in the UK
Advances in Therapy (2019)
-
Clinical Efficacy and Safety of Current Interventions for Choroidal Neovascularization Associated with Rare Diseases: A Systematic Literature Review
Advances in Therapy (2018)
-
Intravitreal anti-VEGF treatment for choroidal neovascularization secondary to punctate inner choroidopathy
International Ophthalmology (2018)