Abstract
Aim
To clarify the 2-year efficacy of ranibizumab for patients with polypoidal choroidal vasculopathy (PCV) with recurrent or residual exudation from branching vascular networks after previous photodynamic therapy (PDT).
Methods
We retrospectively reviewed 26 eyes of 26 Japanese patients (22 men, 4 women) in this pilot study. All eyes had PCV with complete regression of polypoidal lesions resulting from PDT detected by indocyanine green angiography (ICGA), but recurrent or residual leakage from branching vascular networks on fluorescein angiography and evidence of persistent fluid on optical coherence tomography (OCT). Three consecutive intravitreal injections of ranibizumab (0.5 mg/0.05 ml) were administered to all eyes.
Results
The mean logarithm of the minimum angle of resolution best-corrected visual acuity (BCVA) improved significantly from 0.55 at baseline to 0.35 at 12 months (P<0.0001) and 0.43 at 24 months (P=0.0012). The mean increases in the BCVA 12 and 24 months after baseline were 1.95 and 1.23 lines, respectively. The mean central retinal thickness significantly decreased from 295 μm at baseline to 189 μm at 12 months (P<0.0038) and 163 μm at 24 months (P<0.001). The mean numbers of intravitreal ranibizumab (IVR) injections at months 12 and 24, including the initial treatments, were 5.8 and 8.8, respectively. Five (19.2%) eyes had recurrent polypoidal lesions on ICGA at a mean of 15.7 months after baseline. At month 24, OCT showed no exudation in 17 (65.4%) of the 26 eyes. No adverse events developed.
Conclusions
IVR injections maintained or improved the VA and retinal thickness at 24 months in eyes with PCV with recurrent or residual exudation from branching vascular networks after previous PDT.
Similar content being viewed by others
Introduction
Yannuzzi et al1 in 1990 reported that polypoidal choroidal vasculopathy (PCV) is a distinct clinical entity characterized by a branching choroidal vascular network and polypoidal vascular dilation at the border of the network.
The polypoidal lesions and branching choroidal vascular network are seen as occult choroidal neovascularization (CNV) or minimally classic CNV by fluorescein angiography (FA). Indocyanine green angiography (ICGA) clearly shows the abnormal vascular structure, which is important for definitively diagnosing PCV.1, 2, 3, 4 The branching vascular network often appears as a plaque on ICGA and regarded as type 1 CNV.2, 3
PCV is highly prevalent in Asian patients with age-related macular degeneration (AMD) and also develops in Caucasian patients.4, 5, 6, 7, 8 Treatment with photodynamic therapy (PDT) with verteporfin (Visudyne, Novartis Pharma AG, Basel, Switzerland) can occlude the polypoidal lesions in patients with PCV.9, 10 However, the branching vascular network can remain after PDT.11 Retreatments with PDT have been performed in ∼40% of cases due to recurrent or residual exudation despite resolution of the polypoidal lesions during 2 years of follow-up.12
Ranibizumab (Lucentis, Genentech, Inc., South San Francisco, CA, USA) has become an evidence-based therapy for AMD, worldwide, based on the results of major clinical trials.13, 14, 15 However, antivascular endothelial growth factor (VEGF) therapy using bevacizumab (Avastin, Genentech, Inc.) or ranibizumab for PCV has been reported to be less effective for occluding polypoidal lesions.16, 17, 18 We reported that intravitreal ranibizumab (IVR) was more effective than PDT for maintaining and improving VA and the anatomic changes in patients with PCV with recurrent or residual exudation from branching vascular networks.19
The purpose of the current study was to clarify the efficacy of ranibizumab over 24 months for treating patients with PCV who achieved complete regression of polypoidal lesions, but had recurrent or residual leakage from branching vascular networks after previous PDT.
Materials and methods
In this pilot study, we retrospectively reviewed 26 eyes of 26 Japanese patients (22 men, 4 women; age range, 56–84 years; mean±standard deviation, 74.1±6.3 years) followed up for at least 24 months at Fukushima Medical University Hospital. All eyes had PCV with complete regression of polypoidal lesions due to previous PDT applications detected by ICGA, but recurrent or residual leakage from branching vascular networks on FA and persistent fluid on optical coherence tomography (OCT). All patients were followed up for at least 24 months at Fukushima Medical University Hospital. The 6-month results for 22 of the 26 eyes were reported previously.19 The institutional review board/ethics committee at Fukushima Medical University approved the original observational study of AMD and the retrospective comparative analysis performed in the current study.
A clinical diagnosis of PCV was established based on the ICGA findings of polypoidal lesions before the initial treatment. PDT was the initial treatment for all patients. The laser spot size was determined by FA-guided PDT in 15 eyes and ICGA-guided PDT in 11 eyes. ICGA-guided PDT was chosen if the lesion comprised a large hemorrhage or a large serous PED.10 PDT with verteporfin was administered according to the protocol of the Treatment of Age-Related Macular Degeneration with Photodynamic Therapy Study, except for the greatest linear dimension (GLD) in cases with ICGA-guided PDT.20 A 689-nm laser system (Carl Zeiss Meditec, Dublin, CA, USA) was used and 50 J/cm2 energy was delivered with an 83-second exposure time. The GLD was measured in all eyes based on the FA findings.
In the current study, baseline was defined as the period of treatment of residual or new exudative changes due to branching vascular networks after complete regression of polypoidal lesions after PDT.19 All patients had documented visual loss at baseline and were treated with three consecutive monthly intravitreal injections of ranibizumab. After topical anesthesia was applied, IVR (0.5 mg/0.05 ml) was injected 3.5–4.0 mm posterior to the corneal limbus into the vitreous cavity using a 30-gauge needle. After three consecutive monthly intravitreal injections of ranibizumab, follow-up examinations were performed monthly and additional reinjections were administered according to the criteria of the Prospective OCT Imaging of Patients with Neovascular AMD Treated with intraOcular Ranibizumab (PrONTO) study, that is, VA loss of at least five letters of Early Treatment Diabetic Retinopathy Study (ETDRS) scores with OCT evidence of fluid at the macula, increased central retinal thickness (CRT) on OCT of at least 100 μm, a new macular hemorrhage, a new area of classic CNV, or evidence of persistent fluid on OCT 1 month after the previous injection.21 FA and ICGA were performed when new exudative changes or subretinal hemorrhages were seen on fundus examination or OCT after achieving complete regression of the polypoidal lesions. If recurrent polypoidal lesions were seen on ICGA, we administered combined therapy of one IVR injection and PDT (Figure 1).22 The missing data were imputed using the last-observation-carried-forward method and compared for consistency with those obtained using observed data in eyes retreated using PDT.
The diagram shows the retreatment criteria in the current study after month 3 (3 months after treatment). Follow-up examinations, including evaluation of the OCT images, were performed every month until month 24. PRN=pro re nata (as needed according to the criteria during the second year of the PrONTO study).
The exclusion criteria included previous treatment except for PDT for PCV, such as laser photocoagulation, submacular surgery, and transpupillary thermal therapy; glaucoma; tears in the retinal pigment epithelium (RPE); diabetic maculopathy; retinal vascular occlusion; or idiopathic juxtafoveal retinal telangiectasis. We used the best-corrected visual acuity (BCVA) measured with a Japanese standard decimal VA chart and calculated the mean BCVA using the logarithm of the minimum angle of resolution (logMAR) scale. We also converted the decimal BCVA into the ETDRS VA letter scores with a mathematical method as reported previously.23 All patients underwent a standardized examination including slit-lamp biomicroscopy with a contact lens, fundus color photography, FA, ICGA with a fundus camera (TRC-50 FA/IA/IMAGEnet H1024 system, Topcon, Tokyo, Japan), and confocal scanning laser ophthalmoscopy (Heidelberg Retina Angiograph 2, Heidelberg Engineering, Heidelberg, Germany). All OCT examinations were performed using spectral-domain OCT (Heidelberg Spectralis OCT, Heidelberg Engineering) during the follow-up period. The CRT, defined as the distance from the RPE to the inner limiting membrane, was measured at baseline and 3, 6, 9, 12, 15, 18, 21, and 24 months after treatment (months 3, 6, 9, 12, 15, 18, 21, and 24, respectively) using internal caliper software. FA was performed to determine the lesion type, location, CNV activity, and the GLD. ICGA was performed to determine the presence and location of polypoidal lesions and branching vascular networks, and the GLD in cases with ICGA-guided PDT.
Statistical analysis was performed using the Student’s paired t-test to compare the VA and the CRT. P <0.05 was considered statistically significant. We used the logMAR VA scores for statistical analysis.
Results
Table 1 shows the characteristics of and clinical data from the 26 patients (26 eyes) at baseline. All patients were observed for 24 months. The lesions were classified on FA as minimally classic CNV in three eyes and occult with no classic CNV in 23 eyes. At baseline, ICGA showed that the polypoidal lesions regressed completely in all eyes in response to previous PDT applications. However, all eyes had diffuse leakage from the branching vascular networks on FA and evidence of persistent fluid on OCT. The mean GLD of the entire lesion was 4954 μm.
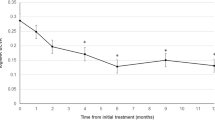
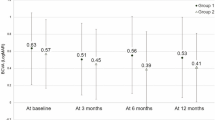
The mean logMAR BCVA levels at baseline and months 12 and 24 were 0.55, 0.35, and 0.43, respectively, indicating significant improvement (P<0.0001, P=0.0012, respectively, by the paired t-test) (Figure 2). The mean changes in the BCVA were improvements of 1.95 and 1.23 lines at months 12 and 24, respectively. Figure 2 shows the distribution of the mean BCVA changes at months 12 and 24. At month 12, 7 (26.9%) of the 26 eyes had an increased BCVA of three lines or more, and 19 eyes (73.1%) had stable VA (defined as a loss of fewer than three lines of vision). At 24 months, five (19.2%) eyes had an increase in the BCVA of three lines or more, and 21 eyes (80.8%) had stable VA (Figure 2). No patient had a decrease in the BCVA of three or more lines during the 24 months.
The graph shows the mean logMAR BCVA (a), the distribution of the mean BCVA changes from baseline (b), and the CRT (c) after treatment with IVR injections for eyes with PCV having recurrent or residual exudation from branching vascular networks after previous PDT. (a) A significant improvement in the mean BCVA is seen at months 12 and 24 compared with baseline (P<0.0001, P=0.0012, respectively, by the paired t-test). Months 12 and 24 are equivalent to 12 and 24 months after treatment. (b) No eyes had decreased BCVA of three lines or more after treatment over 24 months. Months 12 and 24 are equivalent to 12 and 24 months after treatment. (c) A significant decrease in the CRT is seen at months 12 and 24 compared with baseline (P<0.0038, P<0.001, respectively, by the paired t-test).
The mean CRT significantly decreased from 295±174 μm at baseline to 189±110 μm at 12 months (P<0.0038) and 163±106 μm at 24 months (P<0.001) (Figure 2).
At baseline, 18 of the 26 eyes had a serous retinal detachment (SRD); 10 of the 26 eyes had cystoid macular edema (CME); and 6 of the 26 eyes had a retinal pigment epithelial detachment (PED). The SRD resolved in 14 (77.8%) eyes after a mean of 1.9 months post baseline. The CME resolved in eight (80%) eyes after a mean of 2.1 months post baseline. The PED decreased in size in two (33.3%) eyes and remained in four (66.6%) eyes. Figures 3 and 4 show ocular images of patients treated with IVR injections.
Case 17. A 69-year-old man treated with ranibizumab for recurrent exudation from branching vascular networks. (a–d) At the initial treatment, the BCVA is 0.4 decimal VA in the right eye with PCV. (a) A red-free photograph shows lipid and a SRD at the macular area. (b) FA shows occult CNV. (c) ICGA clearly shows polypoidal lesions and a branching vascular network. (d) A horizontal OCT image shows a SRD and anterior protrusion of a highly reflective layer corresponding to the polypoidal lesions. ICGA-guided PDT was applied as the initial treatment (laser spot size, 4900 μm). (e–h) Three months after the initial PDT, the BCVA remains 0.4 decimal VA. (g) ICGA shows complete regression of the polypoidal lesions. (h) A horizontal OCT image shows that the SRD has resolved. No additional treatment is needed. (i–l) Eighteen months after the initial PDT, the BCVA decreased from 0.4 to 0.3 decimal VA. (j) FA shows recurrent leakage at the macular area. (k) ICGA shows a branching vascular network and complete regression of the polypoidal lesions. (l) A horizontal OCT image shows recurrence of the SRD. This period was defined as baseline. Three consecutive monthly IVR injections were performed. (m–p) Three months after baseline, the BCVA improved from 0.3 to 0.5 decimal VA. (n) FA shows no leakage at the macular area. (p) A horizontal OCT image shows that the SRD has decreased in size. No additional treatment was performed. (q, r) Eight IVR injections were administered over 12 months. The BCVA improved from 0.3 to 0.5 decimal VA with a smaller SRD on OCT. No additional treatment is needed. (s–v) At month 24, the BCVA improved from 0.3 to 0.6 decimal VA. (t) FA shows recurrent leakage at the macular area. (u) ICGA shows a branching vascular network and no polypoidal lesions. (v) The twelfth IVR injection was administered.
Case 19. An 80-year-old man treated with ranibizumab for recurrent exudation from a branching vascular network. (a–d) The BCVA is 0.3 decimal VA in the right eye with PCV at the initial treatment. (a) A red-free photograph shows a SRD at the macular area. (b) FA shows occult CNV. (c) ICGA clearly shows polypoidal lesions and a branching vascular network. (d) A horizontal OCT image shows a SRD and anterior protrusion of a highly reflective layer corresponding to the polypoidal lesions. ICGA-guided PDT was applied as the initial treatment (laser spot size, 6300 μm). (e–h) Six months after the initial PDT with two applications of PDT, the BCVA remains 0.3 decimal VA. (g) ICGA shows complete regression of the polypoidal lesions. (h) A horizontal OCT image shows that the SRD has resolved. No additional treatment is needed. (i–l) Twenty-five months after the initial PDT, the BCVA decreased from 0.3 to 0.15 decimal VA. (j) FA shows recurrent leakage at the macular area. (k) ICGA shows a branching vascular network and complete regression of the polypoidal lesions. (l) A horizontal OCT image shows recurrence of the SRD. This period was defined as baseline. Three consecutive monthly intravitreal injections of ranibizumab (IVR) were administered. (m–p) Three months after baseline, the BCVA remains stable. (n) FA shows no leakage at the macular area. (p) A horizontal OCT image shows that the SRD has decreased in size. No additional treatment is needed. (q–t) At month 14, six IVR injections were administered. The BCVA improved from 0.15 to 0.2 decimal VA. (r) FA shows leakage at the macular area. (s, t) ICGA shows recurrent polypoidal lesions with the SRD on OCT. An additional treatment of combined therapy of IVR and PDT was performed. (u, v) At month 24, seven IVR injections and one combined treatment of IVR and PDT were administered. The BCVA improved from 0.15 to 0.6 decimal VA. A horizontal OCT image shows no exudation.
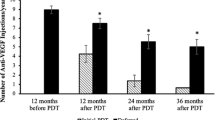
The mean numbers of IVR injections at months 12 and 24, including the initial treatments were 5.8 and 8.8, respectively. In 5 (19.2%) of the 26 eyes, ICGA showed recurrent polypoidal lesions after a mean of 15.7 months post baseline; all five eyes received additional treatment of combined therapy of IVR injections and PDT (Figure 4). At month 24, OCT showed exudation in 9 (34.6%) of the 26 eyes, which were treated with additional IVR injections.
No complications developed, such as increased subretinal hemorrhages (>1 disc diameter), inflammation, intraocular pressure elevations over 21 mm Hg, severe visual loss, endophthalmitis, progression of cataract, or systemic adverse events.
Discussion
The current study showed that IVR injections significantly improved the VA and the anatomic changes without adverse events in patients with PCV with exudative lesions from branching vascular networks after previous PDT during a 24-month follow-up period.
Anti-VEGF therapy with ranibizumab, now considered one of the evidence-based therapies for AMD worldwide, reduced the fluid from PCV, but seems to be less effective for occluding polypoidal lesions.17, 18 However, PDT with verteporfin resolves the polypoidal lesions with high prevalence in PCV eyes.9, 10 However, longer follow-up studies of PDT of 2 or 3 years have reported that the VA decreased at the final visit.24, 25, 26 It is well known that PDT cannot resolve the branching vascular networks, which may cause recurrent or new polypoidal lesions or both.11 We reported that about PDT retreatments were performed in ∼40% of cases due to recurrent or residual exudation despite complete regression of the polypoidal lesions during 2 years of follow-up in patients with PCV.12 Moreover, additional PDT applications in patients with completely regressed polypoidal lesions after PDT, but recurrent or residual exudation from branching vascular networks, were less effective compared with IVR injections during 6 months of follow-up.19 Caution is advised when considering additional treatments for such patients, because additional PDT applications may be less efficacious over the long term. In the current study, IVR injections significantly improved the VA and the anatomic changes at month 24 without adverse events in our patients.
Although no current eyes had polypoidal lesions at baseline due to previous PDT, the polypoidal lesions recurred in five (19.2%) of the 26 eyes after a mean of 15.7 months. Recently, the EVEREST study reported significant efficacy of IVR injections and PDT or PDT monotherapy for achieving complete regression of polyps in patients with PCV compared with ranibizumab monotherapy.27 We also reported the efficacy of ranibizumab plus PDT for patients with PCV for improving VA and anatomic changes at the 12-month follow-up and decreasing retinal and choroidal thicknesses at the 6-month follow-up evaluation.22, 28 Based on these results, we changed our treatment to IVR injections combined with PDT as additional treatment for the five patients with polypoidal lesions and achieved complete regression of the polypoidal lesions. It may be a reasonable strategy to consider adding PDT for recurrent polypoidal lesions when administering ranibizumab monotherapy for patients with residual or new exudative changes in the branching vascular networks despite complete regression of the polypoidal lesions. Therefore, it is important to use ICGA to manage patients with PCV.
In the current study, the mean numbers of injections of ranibizumab at months 12 and 24, including the initial treatments, were 5.8 and 8.8, respectively, which is almost equivalent to that of the PrONTO study (5.0 and 9.9, respectively).21 The increased subretinal hemorrhages after PDT for patients with PCV is a well-known complication with incidence rates from 18 to 30%.10, 11, 20 While the current study had a small number of cases, subretinal hemorrhages did not occur during 24 months.
In conclusion, the current study showed that IVR injections were effective for maintaining or improving VA and the anatomic changes in patients with PCV with recurrent or residual exudation from branching vascular networks without adverse events over 24 months. Long-term follow-up may be more effective if patients with exudation due to recurrent polypoidal lesions are treated with IVR injections plus PDT. Establishing an accurate diagnosis using ICGA is important for evaluating and treating PCV. As the current was a pilot study, further large and long-term prospective randomized studies are needed to determine the efficacy and safety profiles of ranibizumab for patients with PCV.

References
Yannuzzi LA, Sorenson J, Spaide RF, Lipson B . Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990; 10: 1–8.
Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA . Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 1995; 15: 100–110.
Yannuzzi LA, Ciardella A, Spaide RF, Rabb M, Freund KB, Orlock DA . The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol 1997; 115: 478–485.
Maruko I, Iida T, Saito M, Nagayama D, Saito K . Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol 2007; 144: 15–22.
Lafaut BA, Leys AM, Snyers B, Rasquin F, De Laey JJ . Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol 2000; 238: 752–759.
Scassellati-Sforzolini B, Mariotti C, Bryan R, Yannuzzi LA, Giuliani M, Giovannini A . Polypoidal choroidal vasculopathy in Italy. Retina 2001; 21: 121–125.
Ladas ID, Rouvas AA, Moschos MM, Synodinos EE, Karagiannis DA, Koutsandrea CN . Polypoidal choroidal vasculopathy and exudative age-related macular degeneration in a Greek population. Eye 2004; 18: 455–459.
Byeon SH, Lee SC, Oh HS, Kim SS, Koh HJ, Kwon OW . Incidence and clinical 182 patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol 2008; 52: 57–62.
Tano Y, Ophthalmic PDT Study Group. Guidelines for PDT in Japan. Ophthalmology 2008; 115: 585–585.
Saito M, Iida T, Nagayama D . Photodynamic therapy with verteporfin for age-related macular degeneration or polypoidal choroidal vasculopathy: comparison of the presence of serous retinal pigment epithelial detachment. Br J Ophthalmol 2008; 92: 1642–1647.
Akaza E, Mori R, Yuzawa M . Long-term results of photodynamic therapy of polypoidal choroidal vasculopathy. Retina 2008; 28: 717–722.
Saito M, Nagayama D, Iida T . Photodynamic therapy with verteporfin for polypoidal choroidal vasculopathy: long-term results. Nippon Ganka Gakkai Zasshi 2009; 113: 792–799 (in Japanese).
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et alMARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1419–1431.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et alANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1432–1444.
Mitchell P, Korobelnik JF, Lanzetta P, Holz FG, Prünte C, Schmidt-Erfurth U et al. Ranibizumab (Lucentis) in neovascular age-related macular degeneration: evidence from clinical trials. Br J Ophthalmol 2010; 94: 2–13.
Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Oshima Y, Kamei M et al. Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol 2008; 92: 70–73.
Kokame GT, Yeung L, Lai JC . Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: an Interim 6-month report. Br J Ophthalmol 2010; 94: 297–301.
Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K et al. One-year results of three monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in japanese patients. Am J Ophthalmol 2012; 154: 117–124.
Saito M, Iida T, Kano M . Intravitreal ranibizumab for polypoidal choroidal vasculopathy with recurrent or residual exudation. Retina 2011; 31: 1589–1597.
Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP Report 1. Arch Ophthalmol 1999; 117: 1329–1345.
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol 2009; 148: 43–58.
Saito M, Iida T, Kano M . Combined Intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy. Retina 2012; 32: 1272–1279.
Saito M, Iida T, Kano M . Intravitreal ranibizumab for exudative age-related macular degeneration with good baseline visual acuity. Retina 2012; 32: 1250–1259.
Kurashige Y, Otani A, Sasahara M, Yodoi Y, Tamura H, Tsujikawa A et al. Two-year results of photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 2008; 146: 513–519.
Akaza E, Yuzawa M, Mori R . Three-year follow-up results of photodynamic therapy for polypoidal choroidal vasculopathy. Jpn J Ophthalmol 2011; 55: 39–44.
Leal S, Silva R, Figueira J, Cachulo ML, Pires I, de Abreu JR et al. Photodynamic therapy with verteporfin in polypoidal choroidal vasculopathy: results after 3 years of follow-up. Retina 2010; 30: 1197–1205.
Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H et al. EVEREST Study: Efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 2012; 32: 1453–1464.
Maruko I, Iida T, Sugano Y, Saito M, Sekiryu T . Subfoveal retinal and choroidal thickness after verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 2011; 151: 594–603.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Disclaimer
The authors have no proprietary interest in any aspect of this study and received no government funding.
Rights and permissions
About this article
Cite this article
Saito, M., Iida, T., Kano, M. et al. Two-year results of intravitreal ranibizumab for polypoidal choroidal vasculopathy with recurrent or residual exudation. Eye 27, 931–939 (2013). https://doi.org/10.1038/eye.2013.114
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2013.114
Keywords
This article is cited by
-
Intravitreal aflibercept for active polypoidal choroidal vasculopathy without active polyps
Scientific Reports (2019)
-
Prognostic factors after aflibercept therapy for typical age-related macular degeneration and polypoidal choroidal vasculopathy
Japanese Journal of Ophthalmology (2018)
-
High-dose ranibizumab monotherapy for neovascular polypoidal choroidal vasculopathy in a predominantly non-Asian population
Eye (2015)