Abstract
Purpose
To investigate the potential association between glaucoma prevalence and supplemental intake, as well as serum levels of vitamins A, C and E.
Methods
This cross-sectional study included 2912 participants in the 2005–2006 National Health and Nutrition Examination Survey, age ≥40 years, who self-reported a presence or absence of glaucoma. Participants were interviewed regarding the use of dietary supplements during the preceding 30-day period. Participants also underwent serum measurements of vitamins A, C, and E (both alpha- and gamma-tocopherol). Information on the primary outcome measure, presence or absence of glaucoma, as well as demographic information, comorbidities and health-related behaviors, was assessed via interview.
Results
Multivariate odds ratios for self-reported glaucoma, comparing the highest quartile of consumption to no consumption, and adjusted for potential confounding variables were 0.48 (95% confidence interval (CI) 0.13–1.82) for vitamin A, 0.47 (95% CI 0.23–0.97) for vitamin C, and 2.59 (95% CI 0.89–7.56) for vitamin E. Adjusted odds ratios for self-reported glaucoma comparing the highest vs lowest quintiles of vitamin serum levels were 1.44 (95% CI 0.79–2.62) for vitamin A, 0.94 (95% CI 0.42–2.11) for vitamin C, 1.40 (95% CI 0.70–2.81) for alpha-tocopherol, and 0.64 (95% CI 0.24–1.70) for gamma-tocopherol.
Conclusion
Neither supplementary consumption with nor serum levels of vitamins A and E were found to be associated with glaucoma prevalence. While low- and high-dose supplementary consumption of vitamin C was found to be associated with decreased odds of glaucoma, serum levels of vitamin C did not correlate with glaucoma prevalence.
Similar content being viewed by others
Introduction
Glaucoma is a chronic, irreversible optic neuropathy, for which the only confirmed modifiable risk factor is intraocular pressure (IOP). There has been longstanding interest in the potential impact of environmental or lifestyle factors such as diet composition on glaucoma development and progression, either via an effect on IOP, or through more direct mechanisms such as modification of retinal ganglion cell apoptosis.1 Identification of lifestyle factors impacting glaucomatous disease may provide the basis for elucidating novel therapeutic targets and paradigms beyond the tried and true methods of preventing and treating the disease through medical and surgical IOP lowering.
Based upon what is known about antioxidant vitamins, it can be hypothesized that these common dietary supplements may be of interest as potential neuroprotective agents by defending against oxidative stress in the pathogenesis of glaucoma and retinal ganglion cell injury.2, 3 Vitamin E, most widely available in the United States diet as gamma-tocopherol and in most supplements as its most biologically active form, alpha-tocopherol, is an important natural antioxidant with additional putative antiproliferative properties that may potentially be beneficial in the treatment of glaucoma.4 In the eye, vitamin E is regenerated through reactions with vitamin C, another antioxidant found in the aqueous humor.5 Two previously conducted epidemiologic studies, which focused on dietary risk factors for glaucoma, did not yield consistent associations between glaucomatous disease and the intake of antioxidants and vitamin nutrients,6, 7 with subsequent research relating glaucoma to serum vitamin levels being confined to small clinic-based case–control studies.8, 9
The National Health and Nutrition Examination Survey (NHANES), conducted by the Centers for Disease Control and Prevention (CDC), is an annual national population-based study, which provides information regarding the health of the United States population. It includes an extensive interview portion that determines information regarding a variety of health conditions and medication use, as well an objective physical examination component. The 2005–2006 data release of NHANES included measurements of serum levels of several vitamins in addition to a detailed assessment of participants’ use of dietary supplements. We used this extensive, nationally representative sample to study the association between glaucomatous disease and vitamin A, C, and E consumption.
Materials and methods
Sample and population
Publicly available data from the 2005–2006 administrations of the NHANES,10 a cross-sectional series of interviews and examinations of the civilian, non-institutionalized population of the United States, was used to assess the relationship between self-reported glaucoma and the intake of vitamins A, C, and E as determined by interview and confirmed by serum vitamin levels. Overseen by the CDC, NHANES administers interviews and examinations of ∼5000 persons per year in an effort to accumulate US health statistics. This population-based study uses a stratified multistage sampling design that requires a weighting scheme to provide optimal estimates regarding the prevalence of many chronic diseases in the US population.
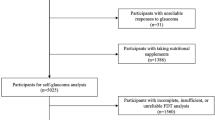
A total of 2934 participants in NHANES between 2005 and 2006 who were 40 years and older and underwent both the interview and examination portions of the study were included in our analysis. An additional 21 participants were excluded because of reported uncertainty regarding whether or not they had previously been diagnosed as having glaucoma, and 1 participant was excluded for not answering the question pertaining to glaucoma diagnosis.
Measures
The intake of vitamins A, C, and E from dietary supplements and antacids served as primary predictor variables. NHANES included a home interview regarding the use of prescription and non-prescription dietary supplements and antacids that had been consumed during the 30-day period before the interview. This information was aggregated to determine the average daily intake for each vitamin from the consumption of dietary supplements and antacids. The population was divided into quartiles of intake for each of the vitamins under study, and an additional ‘no intake’ group was created such that there were a total of five categories of supplemental intake for each vitamin. In addition, NHANES measured the serum levels of Vitamins A, C, E (as alpha-tocopherol and gamma-tocopherol) for participants undergoing a laboratory examination.11 The serum level of each vitamin was also divided into quintiles, and given that there was a very small group with zero serum vitamin levels, a total of five categories of serum vitamin levels were also created for purposes of assessing the association with glaucoma prevalence.
Serum concentrations of vitamins A, alpha- and gamma-tocopherol were measured using high-performance liquid chromatography (HPLC) with photodiode array detection. Serum concentration of vitamin C was measured using isocratic HPLC with electrochemical detection at 650 mV. All serum specimens were collected using standard venipuncture techniques, frozen at −70 °C, and shipped to the Division of Laboratory Sciences, National Center for Environmental Health, and CDC for analysis. Before transport, vitamin C samples were prepared from fresh serum harvested from blood, mixed 1 : 4 with 6% (60 g/l) metaphosphoric acid solution in order to acidify the serum and stabilize the ascorbate before shipment. Samples were collected in morning and afternoon sessions and were not required to be collected in the fasting state.
Two types of quality control (QC) measures were utilized during sample processing. Blind QC specimens were inserted into sample batches before arrival at the lab. These blind QC specimens were prepared at either high or low concentration to emulate the patient samples, with labels identical to those used for patient samples. One blind QC specimen was randomly included in every batch of 20 specimens that were analyzed. In addition, bench QC specimens were prepared from three serum pools representing low, intermediate, and high levels of the analyte in question. These were prepared in the same manner as patient samples and analyzed in duplicate at the beginning and end of each run. Results not meeting strict QC control criteria were not reported. The coefficients of variations ranged from 3.0 to 3.5 for vitamin A, 2.4 to 2.5 for alpha-tocopherol, 2.3 to 2.9 for gamma-tocopherol, and 3.7 to 5.7 for vitamin C.11
The presence or absence of self-reported glaucoma served as the primary outcome variable. Variables considered to be potential confounders before data analysis included age, sex, race, annual household income, and education; health-related behaviors such as smoking (current, past, or never) and alcohol use (number of alcoholic drinks per day over the last year); body mass index (BMI); comorbid medical conditions such as self-reported history of osteoporosis, kidney failure, stroke, thyroid disease, emphysema, liver disease, cancer, congestive heart failure (CHF), diabetes, angina, coronary heart disease (CHD), myocardial infarction (MI), and chronic bronchitis; whether or not the patient was taking treatment for anemia in the prior 3 months; comorbid eye conditions such as self-reported history of cataract extraction, diabetic retinopathy, and macular degeneration; self-reported general health condition (self-rated as excellent or very good, fair, or poor or very poor); and spherical equivalent on objective refraction. Of note, some participants were unable to provide annual household income information within specific ranges. In such circumstances, participants were asked to note whether annual household income was above or below $20 000. Thus, there is a small proportion of participants for whom annual household income is known to be above $20 000, but more detailed information is unavailable.
Data analysis
Design-adjusted Rao-Scott Pearson-type χ2 and Wald tests were used to compare the distribution of possible confounding variables between participants with and without self-reported glaucoma for categorical and continuous variables, respectively. Several multivariate logistic regression models were created by sequential addition of potential confounding variables. These models were used to examine the possible independent association between categories of supplemental vitamin intake, or serum vitamin levels, and self-reported glaucoma. The final multivariate models excluded potential confounding comorbidities not found to be significant at the P≤0.1 level in the multivariate model. These excluded confounders were spherical equivalent on objective refraction; BMI; self-reported macular degeneration; coexistent medical conditions including kidney failure, osteoporosis, stroke, CHF, diabetes, CHD, MI, liver disease, emphysema, and chronic bronchitis; and treatment for anemia in the past 3 months. In an effort to most accurately estimate confidence intervals around point estimates, the analysis was performed in Stata 12.0 (College Station, TX, USA) using weighted data, and SEs of population estimates were calculated using Taylor linearization.
Results
Population characteristics
The 2005–2006 NHANES data yielded 2912 participants aged 40 years and older, who participated in both the interview and examination portions of the study and were able to self-report a presence or absence of glaucoma. Of these participants, 203 self-reported glaucoma, representing 5.42% (SEM 0.5%) of the US population.
Table 1 presents information on demographics, health-related behaviors and comorbidities of the study population, categorized by whether participants self-reported the presence or absence of a glaucoma diagnosis. Among the several demographic characteristics that differed significantly between these two populations was the mean age of those with and without glaucoma, found to be 67.4 years (SEM 1.36 years) and 56.5 years (SEM 0.71 years), respectively (P≤0.0001). Other parameters found to vary between groups included race (P=0.0023), annual household income (P=0.0466), smoking status (P=0.0006), and the average daily number of alcoholic drinks consumed over the past year (P=0.0228). While self-reported general health condition did not differ significantly between the two groups, those who self-reported glaucoma had a significantly higher prevalence of AMD (P=0.0004), diabetic retinopathy (P=0.0004), and history of cataract extraction (P≤0.0001).
Intake of supplemental vitamins A, C, and E
Multivariate logistic regression models were constructed to assess the odds of having a glaucoma diagnosis based upon the supplemental consumption of vitamins A, C, and E (Table 2), while adjusting for potential confounding variables. Each model successively adjusted for additional confounders, with model A adjusting only for age, model B for age and other demographic characteristics, and model C for age, demographic characteristics, health-related behaviors, self-reported general health condition, and comorbidities. Medical and ocular comorbidities were excluded from consideration as confounders if they were not significant at the P≤0.1 level in the multivariate model; notably, no coexistent systemic medical conditions and only one visual comorbidity, self-reported diabetic retinopathy, was retained in the final adjusted model.
Supplemental consumers of vitamin A did not have increased odds of self-reported glaucoma relative to those not consuming vitamin A in any model, whether unadjusted or adjusted for confounders, and regardless of quartile of consumption. Supplemental consumers of vitamin C at the first quartile level (≤100 mg/day) had significantly decreased odds of glaucoma compared to those not consuming supplemental vitamin C in all of the models (model C adjusted odds ratio (OR) 0.34, 95% CI 0.13–0.87); furthermore, this protective effect was also found to be significant at the highest quartile dose of vitamin C (model C adjusted OR 0.47, 95% CI 0.23–0.97). Supplemental consumers of vitamin E at the highest quartile level (>400 IU/day) had significantly higher odds of glaucoma compared with non-consumers in the unadjusted model (OR 3.40, 95% CI 1.58–7.33), which persisted after adjustment for age and demographic characteristics (OR 3.15, 95% CI 1.29–7.72), but not after further adjustment for health-related behaviors and general health condition. Trend analyses showed a significant trend P-value for the vitamin E unadjusted model (P=0.004) with higher doses being associated with a greater prevalence of glaucoma and further adjusted models showed borderline significant P-values for this association. However, significant dose–response relationships were not observed with the other vitamins, in any model.
Serum levels of vitamins A, C, alpha- and gamma-tocopherol
We constructed similar multivariate logistic regression models to compare the odds of glaucoma prevalence among participants based upon their serum levels of vitamins A, C, and E (both alpha- and gamma-tocopherol forms), while adjusting for potential confounding variables (Table 3). For the serum level analyses, model A adjusted for age, model B for age and other demographic variables, and model C for age, demographic variables, health-related behaviors, self-reported general health condition, self-reported thyroid disease, history of cataract extraction, and diabetic retinopathy. As compared with those in the first quintile of serum vitamin A level, participants with higher levels of serum vitamin A were not found to have significantly greater or lesser odds of having been diagnosed with glaucoma. However, there was a significant trend towards greater odds of glaucoma diagnosis with increasing quintiles of serum vitamin A levels in the unadjusted model (P=0.002), which persisted after adjusting for age and demographic characteristics (P=0.032). When all variables were included in the model (model C), this trend was found to remain borderline significant (P=0.052). We did not observe any association between the odds of glaucoma prevalence and serum vitamin C levels, a negative finding, which was confirmed in the trend analysis. Participants found to have the highest quintile of serum alpha-tocopherol levels (>1720 μg/dl) had significantly higher odds of glaucoma compared with those in the lowest quintile (OR 1.59, 95% CI 1.02–2.47) in the unadjusted model. In addition, there was a significant trend of increasing odds of glaucoma with increasing quintiles of serum alpha-tocopherol levels (P=0.009). However, neither of these effects persisted after adjusting for age. Participants with serum gamma-tocopherol levels in the 2nd, 3rd, and 4th quintiles had significantly decreased odds of glaucoma compared with those with gamma-tocopherol levels in the lowest quintile, but only in the unadjusted model.
Discussion
This study of a US national population-based sample of adults aged 40 years and older did not find conclusive evidence that supplementary consumption of vitamins A or E, as determined by self-report or by measurement of serum levels, is related to the self-reported prevalence of glaucomatous disease. We found that those reporting vitamin C supplementation at the first quartile level (≤100 mg/day) and at the fourth quartile level (>900 mg/day) had significantly lower adjusted odds of having been diagnosed with glaucoma than those not receiving vitamin C supplementation, suggesting that vitamin C supplementation may have a protective effect. No dose–response relationship was demonstrated, however, and analysis of serum vitamin C levels showed no beneficial association with regard to glaucoma prevalence.
The results in this analysis of nutrient intake and glaucoma prevalence are consistent with the results of several prior studies of various populations, which also did not find consistent relationships between vitamin intake and glaucomatous disease. Coleman et al7 has investigated the effect of diet on glaucoma incidence among women within the Study of Osteoporotic Fractures. While the investigators found that the consumption of certain foods such as collards, kale, and carrots appeared to be protective against glaucoma, analysis of the constituent nutrients did not reveal a significant protective effect related to the intake of vitamins A, C, or E.7 In an analysis examining the relationship between the consumption of various nutritional components and open-angle glaucoma confirmed by chart review using prospective data from the Nurses’ Health and Health Professionals Follow-up Studies, Kang et al6 also found no appreciable effect of vitamin A intake on the incidence of glaucoma. These investigators, however, did find a statistically significant isolated protective effect in the third quintile of total vitamin C intake,6 somewhat similar to our findings that supplementation with vitamin C at low and high quartile levels conferred decreased odds of glaucoma. Despite appropriate methodological considerations, these spurious findings of an association between Vitamin C intake and glaucoma incidence and prevalence should, at best, be considered with skepticism.
The present study is unique in its large-scale population-based investigation of the relationship between glaucoma and serum vitamin levels. Previous results from a clinic-based case–control study in Japan reported by Yuki et al8 suggested that while serum vitamin C levels were lower in those with normal tension glaucoma than in control subjects without ocular disease, no differences in serum vitamin A or E levels were found in this small study sample. Another clinic-based case–control study by Engin et al9 showed that serum levels of vitamins A and E were increased among patients with glaucoma in their unadjusted analyses. The NHANES data, by providing information on serum vitamin levels, allowed the opportunity to adjust for potential confounding variables. Such an adjustment resulted in no evidence in support of an independent association between glaucomatous disease and vitamin A, C, alpha- or gamma-tocopherol serum levels. It has been recently reported that 1 in 9 glaucoma patients use complementary and alternative medicines, including vitamin supplements, as part of glaucoma therapy, with the majority not disclosing this practice to their ophthalmologists.12 It is well known that vitamin supplementation, particularly in high doses, may be associated with substantial risk that must be balanced with any potential benefit for a given medical condition.13, 14, 15 Our study corroborates prior findings from other studies in concluding that there is no compelling evidence in support of antioxidant dietary supplementation as a means of preventing glaucomatous disease.
We acknowledge that this study has several limitations. Our conclusions are necessarily limited by reliance on self-reporting of disease status, which may be subject to recall bias and disease misclassification. In particular, glaucoma diagnosis should ideally be confirmed by a comprehensive ophthalmologic examination including optic nerve assessment in all such studies. If misclassification of glaucoma diagnosis is non-differential among those consuming high and low levels of antioxidants or among those with high- and low-serum antioxidant levels, the results may be biased towards the null, thereby underestimating the strength of the relationship between antioxidant consumption and glaucoma. The ascertainment of supplement intake information in our study was based upon 30-day recall and thus the data did not allow distinction between participants who had recently begun supplementation and those who had been on long-term supplementation. Furthermore, one cannot exclude the possibility that glaucomatous disease may impact serum antioxidant levels, thereby making it more difficult to assess the impact of vitamin supplementation on the pathogenesis of glaucomatous disease in such a cross-sectional study. However, there is no evidence to support such a hypothesis that glaucoma impacts serum vitamin levels at the present time. Finally, sources of antioxidants from dietary foods rather than supplements were not evaluated in this study; the relationship between glaucoma and total or dietary antioxidant intake may differ from the relationship of this disease to supplementary intake of antioxidants.
In summary, we found no compelling evidence to suggest a relationship between dietary supplementation with vitamins A or E and glaucomatous disease in this large population-based study. There was, however, weak evidence that supplemental vitamin C intake may perhaps be associated with decreased odds of glaucoma, although such a finding should be robustly reproducible in additional studies before therapeutic recommendations. Unlike prior studies examining the relationship between vitamin consumption and glaucoma prevalence, NHANES allowed measurement of serum vitamin levels with adjustment for potential confounding factors, which provided objective evidence supporting the lack of an association between serum vitamin levels and glaucoma. These findings suggest that, based upon all available data at the present time, supplemental antioxidant vitamin intake should not be recommended as a protective strategy for purposes of preventing glaucomatous disease.

References
Pasquale LR, Kang JH . Lifestyle, nutrition, and glaucoma. J Glaucoma 2009; 18 (6): 423–428.
Izzotti A, Bagnis A, Saccà SC . The role of oxidative stress in glaucoma. Mutat Res 2006; 612 (2): 105–114.
Ritch R . Neuroprotection: is it already applicable to glaucoma therapy? Curr Opin Ophthalmol 2000; 11 (2): 78–84.
Engin KN . Alpha-tocopherol: looking beyond an antioxidant. Mol Vis 2009; 15: 855–860.
Lien EL, Hammond BR . Nutritional influences on visual development and function. Prog Retin Eye Res 2011; 30 (3): 188–203.
Kang JH, Pasquale LR, Willett W, Rosner B, Egan KM, Faberowski N et al. Antioxidant intake and primary open-angle glaucoma: a prospective study. Am J Epidemiol 2003; 158 (4): 337–346.
Coleman AL, Stone KL, Kodjebacheva G, Yu F, Pedula KL, Ensrud KE et al. Glaucoma risk and the consumption of fruits and vegetables among older women in the study of osteoporotic fractures. Am J Ophthalmol 2008; 145 (6): 1081–1089.
Yuki K, Murat D, Kimura I, Ohtake Y, Tsubota K . Reduced-serum vitamin C and increased uric acid levels in normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol 2010; 248 (2): 243–248.
Engin KN, Yemişci B, Yiğit U, Ağaçhan A, Coşkun C . Variability of serum oxidative stress biomarkers relative to biochemical data and clinical parameters of glaucoma patients. Mol Vis 2010; 16: 1260–1271.
Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2005. Available at http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. (accessed 19 March 2012).
Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). National Health and Nutrition Examination Laboratory Measurements Protocol. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2005. Available at: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/lab_methods_05_06.htm. and http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/lab_methods_07_08.htm.
Wan MJ, Daniel S, Kassam F, Mutti G, Butty Z, Kasner O et al. Survey of complementary and alternative medicine use in glaucoma patients. J Glaucoma 2012; 21: 79–82.
Fraunfelder FW . Ocular side effects from herbal medicines and nutritional supplements. Am J Ophthalmol 2004; 138 (4): 639–647.
Wilkinson JT, Fraunfelder FW . Use of herbal medicines and nutritional supplements in ocular disorders: an evidence-based review. Drugs 2011; 71 (18): 2421–2434.
Mursu J, Robien K, Harnack LJ, Park K, Jacobs DR . Dietary supplements and mortality rate in older women: The Iowa Women’s Health Study. Arch Intern Med 2011; 171 (18): 1625–1633.
Acknowledgements
This publication was made possible by core grant EY002162 from the National Eye Institute, That Man May See, Inc., Research to Prevent Blindness, and NIH/NCRR/OD UCSF-CTSI Grant number TL1 RR024129. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, S., Singh, K. & Lin, S. Glaucoma and vitamins A, C, and E supplement intake and serum levels in a population-based sample of the United States. Eye 27, 487–494 (2013). https://doi.org/10.1038/eye.2013.10
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2013.10
Keywords
This article is cited by
-
Could the AREDS formula benefit patients with glaucoma?
Eye (2022)
-
Association of serum retinol concentration with normal-tension glaucoma
Eye (2022)
-
The Role of Diet in Glaucoma: A Review of the Current Evidence
Ophthalmology and Therapy (2018)
-
Ascorbic acid concentrations in aqueous humor after systemic vitamin C supplementation in patients with cataract: pilot study
BMC Ophthalmology (2017)
-
Antioxidants and vision health: facts and fiction
Molecular and Cellular Biochemistry (2014)