Abstract
Purpose
To evaluate the association between early and late postoperative intraocular pressure (IOP) and determine if early postoperative IOP can predict the surgical outcome.
Methods
A total of 165 consecutive patients with primary angle-closure glaucoma (PACG) undergoing primary mitomycin-C-augmented trabeculectomy underwent a comprehensive eye examination before surgery and were followed-up on days 1, 7, 14, and 30, and months 3, 6, 12, and 18. IOPs on days 1, 7, 14, and 30 were stratified into groups A (<10 mm Hg), B (≥10 and <15 mm Hg), C (≥15 and <20 mm Hg), and D (≥20 mm Hg). Differences between groups were analyzed using analysis of variance (ANOVA) and Fisher’s exact test. Multivariable regression was used to exam the predictive ability of early IOP for final outcome.
Results
The mean age was 62.5±7.9 years and 41.21% (n=68) were males. Stratified by IOP on days 1, 7, 14, and 30, respectively, mean IOPs at month 18 were different among groups A, B, C, and D (ANOVA, P=0.047, P=0.033, P=0.008, and P<0.001, respectively). Once the IOPs were settled with interventions on day 7 a higher IOP level was associated with decreasing success rate under different outcome definitions, final IOP <15 mm Hg (Fisher’s exact P=0.001) and <20 mm Hg (P=0.039) without medication. Multiple regression showed early IOP predicted final IOP independently from baseline variables. A cutoff value of 13.5 mm Hg on day 7 achieved an accuracy of 80.0 and 57.1% in predicting IOP<15 mm Hg without medication and failure after surgery, respectively.
Conclusions
The IOP at 18 months following primary antifibrotic-augmented trabeculectomy in PACG patients is associated with and predicted by the postoperative IOPs at 1 month. Control of early IOP to 13.5 or less may provide better outcomes.
Similar content being viewed by others
Introduction
Glaucoma is a leading cause of irreversible blindness worldwide.1 Lower intraocular pressure (IOP) is the only known crucial factor that protects visual field over time.2, 3, 4, 5 Filtration surgery remains an important and effective intervention to achieve this goal.6, 7, 8 Eye specialists have taken considerable efforts in performing the surgery for a controllable outcome and IOP has become the surrogate factor in judging surgical success.6, 7, 8, 9 Existing evidences suggest an association between early postoperative IOP and long-term effectiveness of trabeculectomy.10, 11, 12, 13 Long-term success might be predicted by early postoperative IOP with sensitivity from 89 to 93%.11, 12 Accordingly, it has been suggested that a higher IOP after the first postoperative week may require early intervention to ensure success.14 On the contrary, others have reported only a weak association between early and final IOPs and conclude that early postoperative IOPs had limited ability to predict long-term outcomes.15, 16 Until now, the relationship between early and late postoperative IOP was not clarified. We need studies with reasonable sample size and adequate duration of follow-up to conclude the predictive value of early IOP.
Using data from a prospective randomized controlled trial (RCT) of releasable sutures, we re-examined the association between early postoperative and final IOP, and the ability of early postoperative IOPs to predict the outcome of trabeculectomy in Chinese patients with primary angle-closure glaucoma (PACG).
Materials and methods
These data were part of a RCT conducted in four clinical centers of the Tongren Eye Hospital Beijing; the Handan 3rd Hospital (Hebei Province, China); the Anyang Eye Hospital (Henan Province, China); the Fushun Eye Hospital (Liaoning Province, China), and the Chenzhou Eye and Optometry Center (Hunan Province, China). The primary purpose of the trial was to compare the efficacy and complications of trabeculectomy with or without releasable suture in eyes with PACG. Ethics approval was obtained from institutional review boards at the Beijing Tongren Hospital, Capital Medical University, and at all sites. Registration information is publicly available at WHO Primary Clinical Trial Registry (ChiCTR-TRC-00000218).
PACG was defined as primary angle-closure with glaucomatous optic neuropathy and comparative visual field defects in the absence of secondary causes.17
Eligible subjects were recruited from the four centers between April 2006 to November 2007. The inclusion criteria were: (1) age 40 years or above; (2) presence of peripheral anterior synechia (PAS) in the angle for 6 or more clock-hours; (3) IOP ≥21 mm Hg; (4) evidence of glaucomatous optic neuropathy (combination of cup: disc ratio ≥0.7, asymmetry of cup: disc ratio ≥0.2, retinal nerve fiber layer defect, rim: disc ratio <0.1 and notches; (5) corresponding glaucomatous visual fields defect; and (6) axial length ≥21 mm.
The exclusion criteria were: (1) secondary angle-closure; (2) patients with serious systemic diseases or family issues that could preclude follow-up; (3) history of previous intraocular surgery or trauma; and (4) unwillingness to participate.
On obtaining written informed consent, patients were randomized to receive trabeculectomy (Trab arm) or trabeculectomy adjunct with releasable suture (RS arm).
Surgical techniques
All trabeculectomies were carried out by glaucoma specialists using a standardized technique. None of the patients underwent combined cataract and trabeculectomy surgery. Surgery was performed under surface anesthesia. The eye was prepared by aseptic technique and draped with an occlusive dressing to isolate lash follicles. A lid speculum was placed and a 7/0 superior rectus muscle traction suture was placed for ocular stabilization. All operations were performed using a limbus based conjunctival flap. A 10-mm incision was made through conjunctiva and tenons capsule approximately 8–10 mm from the limbus and dissected anteriorly to the limbus. Hemostasis was achieved using monopolar diathermy and a 4 × 3 mm2 rectangular scleral flap partial thickness fashioned. Cellulose sponges soaked in MMC (0.3 mg/ml) were applied under the scleral flap, conjunctiva and tenons for 1–4 min based on the preoperative risk factors. Care was taken to avoid contact with the free edges of the conjunctiva and irrigation with balanced salt solution was performed to wash out any residual MMC solution. A paracentesis was created before removing a 2 × 1.5 mm trabeculectomy block.
Two 10/0 nylon sutures were placed in the posterior corners closing the scleral flap using a releasable technique in the RS arm; fixed sutures were used in the Trab arm. The suture tension was tailored to achieve ooze of aqueous through the scleral flap without shallowing of the anterior chamber. The conjunctival incision was closed with a single running 10/0 vicryl suture and integrity of wound closure checked by irrigating the AC through the paracentesis using balanced salt solution. Any leak from the paracentesis was closed with a single 10/0 nylon suture. Tobramycin 10 mg+4 mg of betnesol was injected subconjunctivally inferiorly and a pad and shield placed on the eye.
The release of the first suture was performed when the IOP was >21 mm Hg with a shallow bleb. All second sutures were released within 10 days of the first suture. Although ophthalmologists were required to follow the protocol, they had the discretion to manage the individual patient.
Examinations
All patients underwent a comprehensive ophthalmic examination at baseline including refraction, static and dynamic Goldmann gonioscopy, Goldmann applanation tonometry, slit lamp and fundus examination, and automated perimeter (Humphrey Field Analyzer 750 (HFA, Humphrey Inc., San Leandro, CA, USA), SITA Fast strategy, 24-2 threshold test were used in clinical centers of Handan, Fushun, and Chenzhou, whereas Oculus Centerfield Type 56950 (Oculus Poland, Ltd., Warsaw, Poland) was used in Anyang Eye Hospital). Visual acuity (LogMAR vision chart, Tumbling 'E' Acuity Sight Test 4 Meters cat. no. 2305 (Precison Vision, La Salle, IL, USA)), applanation tonometery, slit lamp evaluation and assessment of the optic nerve and macula were performed on postoperative days 1, 3, 7 and at 2 weeks, 4 weeks, 3 months, 6 months, 12 months, and 18 months. Additional visits were scheduled if indicated. Use of medication, additional surgery, and complications were recorded. All clinical data were documented in standard forms.
IOP was measured twice by a clinical technician at each study center. If the measurements differed significantly (>2 mm Hg) a third measurement was performed and the mean of the results was recorded.
Statistical methods
Patients with severe postoperative complications (bleb leakage, persistent choroidal detachment), and those who received additional intraocular interventions for non-glaucoma causes were excluded from this study.
Statistical analysis was carried out with SAS v8 (SAS Institute Inc., Cary, NC, USA). For analysis purposes, we looked at different cutoff points. Accordingly success was variously defined as postoperative IOP <15, <18, or <20 mm Hg without medications before a repeat trabeculectomy. Surgical failure was also defined according to the particular analysis as postoperative IOP ≥15, ≥18, and ≥20 mm Hg, introduction of medications for glaucoma or need for a repeat trabeculectomy.
If both eyes of a patient were eligible, the study eye was randomly selected. To investigate the association between early IOPs and IOP at month 18, patients were arbitrarily divided into four groups based on IOP on days 1, 7, 14, 30, respectively: A (<10 mm Hg), B (≥10 and <15 mm Hg), C (≥15 and <20 mm Hg), and D (≥20 mm Hg). IOPs of each group were compared using one-way analysis of variance (ANOVA). Differences in and the effects of early IOPs on the proportion of IOP control among groups at month 18 were tested using Fisher’s exact test. Receiver operating characteristic (ROC) curve was used to determine the best cutoff for outcome prediction. The relative risk (RR) of failure was estimated for the early postoperative IOP above the cutoff. Multivariable regression models were used to predict the final IOP. Alpha error was set to 0.05.
Results
Patients and demographics
A total of 175 subjects were recruited from the clinical trial. Ten patients were excluded because of the following reasons: two patients (1.1%) with choroidal detachment that persisted for more than a month, six patients (3.4%) with bleb leaks, and two (1.1%) who needed cataract surgery within 12 months. The remaining 165 cases were eligible for analysis (Table 1). The past highest recorded IOP in these patients was 46±14.8 mm Hg. In all, 141 (85.2%) patient completed follow-up at month 18. IOPs before the use of postoperative ocular hypotensive drugs (18, 10.9%), suturelysis (1, 0.6%) or further filtering surgery (6, 2.4%) were analyzed and the rest of IOP readings were treated as missing values in regression models. By postoperative day 30 (median 7 days, range 2–68) 98.9% of eyes completely removed the sutures. One patient underwent laser suturelysis at 3 months in Trab arm.
During the 2 weeks following surgery, 14 patients experienced transient hypotony (<5 mm Hg). None of the patients developed maculopathy and IOPs in all these cases returned to >5 mm Hg by 1 month and thereafter. No case developed hypotony during the rest of the follow-up period.
Relationship between early IOPs and surgical outcomes
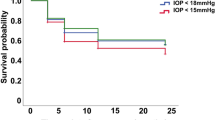
The participants were classified into four early IOP (A, B, C, and D) groups as detailed earlier and success rate by different final IOP cutoffs were calculated and tested (Table 2). Although some of the P-values were not significant when success was defined as final IOP <18 or 20 mm Hg most of the P-values were marginal and a clear trend was observed. A lower early IOP was associated with a higher success rate. There were statistically significant differences amongst the mean final IOPs of the four groups stratified by IOP on days 1 (ANOVA, P=0.047), 7 (P=0.033), 14 (P=0.008), and 30 (P<0.001), respectively (Figure 1). In the Trab arm, mean final IOPs raised from groups A to D stratified by IOP on days 1 (ANOVA, P=0.286), 7 (P=0.009), 14 (P=0.007), and 30 (P=0.079), respectively. It was possible for patients to move between the groups.
Multiple linear regression were performed to explore the effects of early postoperative IOPs (on days 1, 7, 14, and 30) and baseline variables, such as age, PAS, best-corrected visual acuity (BCVA), anterior chamber depth (ACD), vertical cup/disc ratio (VCDR), and visual field mean deviation (MD), on final IOP (Table 3). Among the variables age, BCVA, PAS, ACD, VCDR, MD, and IOP on day 1 did not achieve significance in univariable model. Early IOPs on days 7, 14, and 30 were selected for further modeling. We also include other biological plausible factors in the multivariable regression model, such as age and PAS. Final IOP was regressed on early IOPs of each time point (respectively) and other selected variables (Table 4). PAS was eliminated in the multivariable regression models and the equation for final IOP prediction was constructed. For all of the PACG patients received trabeculectomy either with fixed or releasable sutures: final IOP (mm Hg)=17.3+0.21 × IOP on day 7 (mm Hg)−0.09 × age (years). For PACG patients received trabeculectomy only with fixed sutures: final IOP=17.0+0.36 × IOP on day 7−0.11 × age.
In the multivariable equations IOPs on postoperative days 7, 14, and 30 were the only variables independent from age (in all of the models, P>0.05) and PAS (in all of the models, P>0.05) that significantly affect final IOP. In the RS arm, significant association was not observed until day 30 (B=0.22, P=0.03). For the PACG patients without releasable sutures, 1 mm Hg of rise in IOP at days 7, 14, or 30 predicted an increase of 0.35, 0.23, or 0.47 mm Hg in IOP at one and a half year later.
Outcome prediction
(ROC curve was used to determine the best cutoff for outcome prediction. The area under ROC (AUC) of IOP at day 7 for the three outcome cutoffs (final IOP, 15, 18, and 20 mm Hg ) were 0.661 (P=0.001), 0.596 (P=0.084), and 0.642 (P=0.027), respectively. A cutoff value at 13.5 mm Hg on day 7 achieved an accuracy of 80.0% in predicting success of IOP<15 mm Hg without medication and 57% in predicting failure after surgery, respectively. If the IOP on day 1 was >10.5 mm Hg or the IOP on day 7 was >13.5 mm Hg the RR of failure, which was defined as IOP equal to or greater than 20, were 4.2 (95% CI 1.2–14.8). The RR, if failure was defined as IOP equal to or greater than 15 mm Hg at 18 months, became 4.3 (95% CI 2.1–9.1).
It was also observed that during the 18 months follow-up patients with lower early IOPs (≤18 mm Hg at day 1) required a lower number of anti-glaucoma interventions (P=0.028, RR 3.2, 95% CI 1.2–8.4) and medications (P=0.051, RR 2.8, 95% CI 1.0–7.8) than those with higher early IOPs.
Effects of interventions on outcome
Among patients with the surgical success (≤18 mm Hg) at 18 months, 54% had experienced an IOP increase (>15 mm Hg) within 7 days of surgery. Interventions other than suture release were needed in 8.3, 8.3, and 8.8% of those with high IOP (>15 mm Hg) at days 1, 7, and 14 during the whole follow-ups. The interventions included laser suturelysis, massage at the edge of the scleral flap, needling, medication, and repeat trabeculectomy if needed. The rate of surgical success showed no difference between subgroups with and without interventions (including suture release; Fisher’s exact test, P=0.582, 0.388, and 0.636).
Conclusion
Using post hoc analysis of prospective data from RCT, we found that early IOP following trabeculectomy was associated with the final IOP. Among subgroups with different early IOP levels on days 1, 7, 14, and 30, increasing early IOP indicated a higher final IOP, and, patients with a lower early IOP had a higher success rate.
Previous studies have attempted to use early postoperative IOP to predict a favorable outcome following surgery.10, 11, 12, 14, 16 A retrospective analyses of 92 glaucoma patients with antimetabolite-augmented filtering surgery suggested that 16 mm Hg on day 1 predicted long-term success (≤21 mm Hg without medication) and failure with an accuracy of 88.8 and 55.5%, respectively.12 Another retrospective assessment involved a 2-year follow-up on 203 eyes undergoing unaugmented trabeculectomy.11 Success was defined as an IOP <21 mm Hg at 2 years. In all, 92.6% of successes could be predicted by a first postoperative day IOP of <17 mm Hg; 42.6% of failures could be predicted by an IOP above this level. In this study, ROC curves provided similar results as prior reports. IOP from days 1 to 30 clearly had a maximum area under ROC of 0.71. A cutoff value at 13.5 mm Hg on day 7 achieved an accuracy of 80.0 and 57.1% in predicting success (<15 mm Hg without medication) and failure (≥15 mm Hg or start medication) after surgery, respectively.
Others have found that early postoperative IOP cannot predict final IOP in technically successful glaucoma surgeries. They felt that IOP at the 1-month point is a better indicator for the long-term course and further intervention.15 Although this study pooled data from trabeculectomy as well as combined surgery, and postoperative interventions were not considered in the analysis.15
Manipulation of releasable sutures is believed to substantially affect the filtering pathway. In RS arm, IOPs started showing correlations with final IOP only after 30 days of time (98.9% of eyes completely removed the sutures). In contrast, the IOPs after day 7 in Trab arm correlated well with the 18-month IOP. Suture release could reshape filtering pathway reducing IOP to a wide range of level. Thus, in RS arm it altered the assumed association. In general, the longer the postoperative follow-up, the stronger the association between early postoperative IOP and final IOP. The increasing R2 value with follow-up time in multivariable regression model gives the impression that artificially created filtration gradually becomes stable and finally determined the long-term IOP. Moreover, regardless of difference in success criteria early IOPs were predictive of rates of final success. We also noticed that the immediate interventions after surgery may have a considerable role in maintaining functioning filtering structures and change the probability of success. This is in accordance with Marquardt’s report that intensive postoperative care can significantly increase the proportion of filtering blebs achieving target IOP without medication.18 However, the R2 is small, indicating the process of wound healing for trabeculectomy was complex. Age and PAS did not affect final IOP.
There has been a lot of discussion on setting target IOP.3, 5, 19, 20 In our study, although we did not conclude a specific IOP the trend test supported the idea that patients with lower early postoperative IOP would have a higher probability to success regardless of the criteria. In addition, IOP exhibited increasing importance in guiding the interventions.
The results may have important implications for the management of glaucoma patients and suggests maintaining a lower level of IOP during immediate postoperative period. It could be argued that such an apparent difference in outcome based on early IOPs should encourage surgeons to undertake postoperative interventions to lower down early postoperative IOP. With the current data, we are not able to suggest a lower limit for the IOP control. Lower initial IOP and the measures used to achieve them are not without risk and giving our inability to lower IOP predictably, striving for very low IOPs might not be advantageous.15 A balance must be sought between the risks of inducing hypotony and ensuing complications and the attainment of a safe IOP facilitating long-term successful surgery.10
Analysis of factors contributing to postoperative complication is limited in this study because of a relatively low incidence. It is also important to emphasize that this study did not assess bleb morphology in the early postoperative period. Optimal early bleb characteristics might predict intermediate IOP success; the IOP correlations in our study could be simply potential markers for this. In addition, our follow-up was intermediate in duration (18 months) with some loss to follow-up, and one must be cautious of extrapolating these trends to the long term.
Strictly speaking the results presented are only applicable to only the trabeculectomies in PACG patients with no early leak, choroidal detachment, and so on. However, the demographics of the subjects bear many resemblances with the population enrolled by Sihota.21, 22 Definition of success varies between reported series but quoted success rates ranged between 80 and 100% over 1 year23 are comparable with our results.
In summary, in PACG patients undergoing mitomycin-C-augmented trabeculectomy a low IOP in the first postoperative month was predictive of intermediate term success. Achieving an appropriate postoperative IOP level by this time is associated with less frequent postoperative interventions and higher rate of intermediate successful outcome.

References
Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004; 82: 844–851.
The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 2000; 130 (4): 429–440.
O'Brien C, Schwartz B, Takamoto T, Wu DC . Intraocular pressure and the rate of visual field loss in chronic open-angle glaucoma. Am J Ophthalmol 1991; 111 (4): 491–500.
Stewart WC, Chorak RP, Hunt HH, Sethuraman G . Factors associated with visual loss in patients with advanced glaucomatous changes in the optic nerve head. Am J Ophthalmol 1993; 116 (2): 176–181.
Vogel R, Crick RP, Newson RB, Shipley M, Blackmore H, Bulpitt CJ . Association between intraocular pressure and loss of visual field in chronic simple glaucoma. Br J Ophthalmol 1990; 74 (1): 3–6.
AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 9. Comparison of glaucoma outcomes in black and white patients within treatment groups. Am J Ophthalmol 2001; 132 (3): 311–320.
Parrish RK, Feuer WJ, Schiffman JC, Lichter PR, Musch DC, CIGTS Optic Disc Study Group. Five-year follow-up optic disc findings of the collaborative initial glaucoma treatment study. Am J Ophthalmol 2009; 147 (4): 717–724 e1.
Musch DC, Gillespie BW, Lichter PR, Niziol LM, Janz NK, CIGTS Study Investigators. Visual field progression in the Collaborative Initial Glaucoma Treatment Study the impact of treatment and other baseline factors. Ophthalmology 2009; 116 (2): 200–207.
Birchall W, Bedggood A, Wells AP . Do scleral flap dimensions influence reliability of intraocular pressure control in experimental trabeculectomy? Eye 2007; 21 (3): 402–407.
Hara T, Araie M, Shirato S, Yamamoto S . Conditions for balance between lower normal pressure control and hypotony in mitomycin trabeculectomy. Graefes Arch Clin Exp Ophthalmol 1998; 236: 420–425.
Downes SM, Mission GP, Jones HS, O’Neill EC . The predictive value of post-operative intraocular pressures following trabeculectomy. Eye 1994; 8: 394–397.
Alwitry A, Moodie J, Rotchford A, Abedin A, Patel V, King AJ . Predictive value of early IOP in mitomycin-C augmented trabeculectomy. J Glaucoma 2007; 16: 616–621.
Sukhija J, Jain AK . Early vs late intraocular pressure following trabeculectomy with releasable suture in advanced glaucoma. Ann Ophthalmol (Skokie) 2006; 38: 127–130.
Asamoto A, Yablonski ME, Matsushita M . Predicting long-term results of trabeculectomy from early postoperative intraocular pressure levels. Ophthalmic Surg Lasers 1996; 27 (5): 355–360.
Polikoff LA, Taglienti A, Chanis RA, Ramos-Esteban JC, Donas N, Tsong J et al. Is intraocular pressure in the early postoperative period predictive of antimetabolite-augmented filtration surgery success? J Glaucoma 2005; 14 (6): 497–503.
Stewart WC, Pitts RA . Postoperative prognostic indicators following trabeculectomy. Acta Ophthalmol (Copenh) 1993; 71 (6): 733–738.
American Academy of Ophthalmology. Preferred Practice Patterns. Available at: http://one.aao.org/CE/PracticeGuidelines/PPP.aspx. (Accessed 10 November 2011.
Marquardt D, Lieb WE, Grehn F . Intensified postoperative care versus conventional follow-up: a retrospective long-term analysis of 177 trabeculectomies. Graefes Arch Clin Exp Ophthalmol 2004; 242 (2): 106–113.
Aoyama A, Ishida K, Sawada A, Yamamoto T . Target intraocular pressure for stability of visual field loss progression in normal-tension glaucoma. Jpn J Ophthalmol 2010; 54 (2): 117–123.
Lazaro C, Garcia-Feijoo J, Castillo A, Perea J, Martinez-Casa JM, Garcia-Sanchez J . Impact of intraocular pressure after filtration surgery on visual field progression in primary open-angle glaucoma. Eur J Ophthalmol 2007; 17 (3): 357–362.
Sihota R, Gupta V, Agarwal HC . Long-term evaluation of trabeculectomy in primary open angle glaucoma and chronic primary angle closure glaucoma in an Asian population. Clin Experiment Ophthalmol 2004; 32 (1): 23–28.
Sihota R, Sood A, Gupta V, Dada T, Agarwal HC . A prospective longterm study of primary chronic angle closure glaucoma. Acta Ophthalmol Scand 2004; 82 (2): 209–213.
Raina UK, Tuli D . Trabeculectomy with releasable sutures: a prospective, randomized pilot study. Arch Ophthalmol 1998; 116 (10): 1288–1293.
Acknowledgements
We thank Dr Lan Ping Sun, the vice president of Handan 3rd Hospital, for the logistic support provided by the hospital. We also thank Dr Tian Cai Ye, professor of ophthalmology, Zhongshan Ophthalmic Center, for their valuable advices to develop the study protocol and case report form. We also thank the coordinators: Mrs Ying Luo, in Angyang Eye Hospital, Anyang, Henan Province; Dr Li Xia Guo, Dr Hong Yu Cui and Dr Zhi Hong Zhang at Department of Ophthalmology, Handan 3rd Hospital, Hebei Province. This work was funded by the National ‘Eleventh Five-Year’ Science and Technology Program, the Ministry of Science and Technology of the PRC grant no. 2007BAI18B08.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rong, S., Feng, M., Wang, N. et al. Can early postoperative intraocular pressure predict success following mitomycin-C augmented trabeculectomy in primary angle-closure glaucoma. Eye 27, 403–409 (2013). https://doi.org/10.1038/eye.2012.291
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2012.291
Keywords
This article is cited by
-
Efficacy and safety at 6 months of the XEN implant for the management of open angle glaucoma
Scientific Reports (2020)
-
One-month IOP in mitomycin C-augmented trabeculectomy can predict long-term IOP control in chronic primary angle-closure glaucoma
International Ophthalmology (2019)