Abstract
Background/aims
The purpose of this study was to critically evaluate the Catatrac device as a potential tool for rapid cataract screening in the developing world.
Methods
Patients attending the day case unit at Gartnavel General Hospital for routine cataract surgery were recruited into the study, and divided into two groups: those with mild cataracts with LogMAR acuities <0.48, and those with advanced cataracts with LogMAR acuities ≥0.48. The subjects were examined without pharmacological dilation in a dimly lit room independently by two nurses. Each patient was then examined by an ophthalmologist with a slit lamp, after dilation. If present, cataracts were graded objectively according to the LOCS III classification system.
Results
One hundred and twenty-two eyes of 73 patients were screened for the presence or absence of cataract using the Catatrac device. Thirty-nine eyes had mild cataracts, 43 eyes had advanced cataracts, and there were 40 control eyes with no cataracts. For detecting advanced cataracts, the two nurses using the Catatrac device had a specificity of 95.0%, a sensitivity between 86.0 and 93.0%, and κ values between 0.81 and 0.88 for agreement with slit lamp assessment. For detecting mild cataracts the two nurses using the Catatrac device again had a specificity of 95%, sensitivity of 71–84.6%, and κ values between 0.67 and 0.80 for agreement with slit lamp assessment. Interobserver agreement between the two nurses had a κ value of 0.61 for mild cataract and 0.74 for advanced cataract.
Conclusion
The Catatrac device has a high specificity, sensitivity, and interobserver agreement for advanced cataracts. Although having a slightly lower sensitivity for mild cataracts, the authors believe that this study has demonstrated that it may be a low cost and easy to use device for rapid screening of visually significant cataracts in the developing world.
Similar content being viewed by others
Introduction
Cataract is the leading cause of blindness worldwide, accounting for 47.8% of all causes of blindness.1 The World Health Organisation estimates that 20 million people are blind from bilateral cataracts globally,2 with >90% of the world’s visually impaired living in developing countries.3 In these countries, blindness has significant economic and social consequences resulting in considerable disability and mortality.4
One of the first steps in correcting this problem is with case finding. Several studies have shown that cataract screening programmes in the community are effective in identifying suitable patients for surgery.5, 6, 7, 8 However, these screening programmes are usually carried out by the medical staff, and a shortage of eye surgeons worldwide makes this difficult. In most of Africa, for example, there is only one ophthalmologist per million population.9 Primary health care workers are better placed to screen opportunistically for cataracts in their communities, but a major stumbling block is the need for training and access to expensive equipment such as direct ophthalmoscopes, slit lamp biomicroscopes, and drops to dilate pupils. A cheap and easy screening test for cataracts, which could be performed by non-ophthalmic health care workers, may therefore be of great benefit. The Catatrac device (Catatrac Ltd, Argyll, UK; Figure 1) has been developed to screen cataracts based on fundamental optical principles. It is safe (CE marked and FDA approved), and is marketed as being cheap to produce and easy to use with minimal training. The device is made of low-cost materials such as plastic and rubber. The optics of the device are illustrated in Figure 2.
A red light-emitting diode (LED) is used to create a bright light source. The light from the LED is collimated by a plastic lens, and then passes through a pin hole that acts as an aperture. A second plastic lens creates an image of the pinhole at the patient, after the illuminating beam has been redirected 90° by a prism.
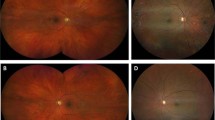
Unlike a direct ophthalmoscope, there are no lenses to select: the user simply presses a button on the shaft of the device, and then views the red reflex created from the patient’s eye to determine whether a cataract exists or not (Figure 3).
This device has the potential to be an easier and cheaper alternative to traditional cataract screening methods that involve expensive equipment, and may be used by non-ophthalmic health care workers. The effectiveness of this instrument has, however, not been validated against conventional cataract diagnostic techniques. The purpose of this study was therefore to critically evaluate the Catatrac device as a potential tool for screening cataracts in the developing world.
Materials and methods
This study was carried out at the Tennent Institute of Ophthalmology in Glasgow, UK. The study followed the tenents of the Declaration of Helsinki, and was approved by the West of Scotland Research Ethics Committee. After informed consent was obtained, patients attending the day case unit at Gartnavel General Hospital for routine cataract surgery were recruited into the study, and were divided into two groups: those with mild cataracts with LogMAR acuities <0.48, and those with advanced cataracts with LogMAR acuities ≥0.48. A group of control patients without cataract were also recruited. Only patients without vitreoretinal pathology were included in the study. Participants were examined for the presence or absence of cataract independently by two ophthalmic nurses using the Catatrac device. The subjects were examined without pharmacological dilation in a dimly lit room. Each nurse was given a solitary, 45-min training session to familiarise themselves with the device before the screening studies commenced. The nurses had no prior contact with the patients, and were not aware of their cataract status or visual acuity. Each patient was then examined for a third time by an ophthalmologist with a slit lamp, after dilation with tropicamide 1% and phenylephrine 2.5%. The presence or absence of cataract was then confirmed by the ophthalmologist and, if present, was graded objectively according to the LOCS III classification system.10
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Statistical method
The specificity and sensitivity of the Catatrac device was determined for mild and advanced cataracts in comparison with standard dilated slit lamp examination by an ophthalmologist. The κ values were calculated to determine the level of agreement between the Catatrac device and the slit lamp as well as interobserver variability between nurses.
Results
One hundred and twenty-two eyes of 73 patients (mean age 67; 40% male) were screened for the presence or absence of cataract using the Catatrac device. Thirty-nine eyes had mild cataracts (LogMAR <0.48), 43 eyes had advanced cataracts (LogMAR ≥0.48), and there were 40 control eyes with no cataracts.
The distribution of cataract subtypes within the entire study population according to the LOCS III classification system is shown in Table 1.
For mild cataracts, the Catatrac device had a specificity of 95.0% for both nurses, and a sensitivity of between 71.7% (nurse 1) and 84.6% (nurse 2). The κ values of 0.80 (SE 0.07) and 0.67 (SE 0.08) were found between the Catatrac device and the slitlamp for nurse 1 and nurse 2, respectively. Interobserver agreement between nurse 1 and nurse 2 had a κ value of 0.61 (SE 0.09).
For advanced cataracts, the Catatrac device again had a specificity of 95.0% for both nurses, and a sensitivity of 86.0 (nurse 1) and 93.0% (nurse 2). The κ values of 0.88 (SE 0.05) and 0.81 (SE 0.06) were found between the Catatrac device and the slitlamp for nurse 1 and nurse 2, respectively. Interobserver agreement between nurse 1 and nurse 2 had a κ value of 0.74 (SE 0.07).
Discussion
Most epidemiological studies have relied on standardised examinations by ophthalmologists to detect the presence and the type of cataract.11, 12, 13 Equipment such as slit lamps have been used, as well as more basic equipment such as torches, and × 2.5 loupes.8, 14 The availability of an accurate and cheap screening tool that could be easily used by non-ophthalmologists would potentially be of great use in cataract detection.
With only one button to switch on the LED light source, and no requirement to change lenses, the Catatrac device is easy to use, with only minimal training required. The device is ergonomic and user friendly, made of a robust but soft touch rubber that feels comfortable in the hands. All of the nurses involved in this study felt comfortable in using the device within 45 min of use. A direct ophthalmosope or slit lamp, in comparison, requires much more rigorous training to develop an acceptable level of competence. The examiner requires only to position the device before the subject’s eye, press the button to switch on the LED light source, and then view. Cataract assessment using this device therefore takes seconds.
The device is also cheap to purchase, costing $60 (USA) to buy new. This compares favourably with a direct ophthalmoscope, which can cost upwards of $200 (USA), or a slit lamp, which would cost several thousand dollars. A direct opthalmoscope is, however, more readily available in an ophthalmology setting.
Our results show that the Catatrac device has a high specificity of 95% for both mild and advanced cataracts. This is an important property of a screening test, which minimises potential over-referral of cataracts to the hospital eye service. The device trades this high specificity against all cataract types with a lower sensitivity for mild cataracts. When mild cataracts were screened the sensitivity dropped to 71.7–84.6%, with a higher disagreement between observers. This suggests that the Catatrac device would be underdetecting mild cataracts. Other studies from the literature have also shown that interobserver agreement is much harder to obtain in the detection of early lens opacities than with more severe cataracts.13, 15
Most of the mild cataracts in the study were of mixed type, and this makes any meaningful analysis of cataract subtype difficult. However, of the LOCS III cataract subclassifications, the highest cataract grade for mild cataracts was found in the nuclear colour group. This may suggest that the device may be weakest at detecting mild nuclear sclerotic cataracts.
However, we found substantial agreement between observers with high sensitivity of 86–93% when screening more advanced cataracts. We chose a LogMAR acuity of 0.48 (Snellen equivalent 6/18) as our cutoff point for advanced cataracts, and several other studies have suggested that this is the optimal level for case detection.16, 17, 18 Several reports of cataract surgical coverage have reported that only advanced cataracts with visual acuities worse than 6/60 are operated on in the developing world.19, 20, 21, 22, 23 It could therefore be argued that the lower sensitivity of the Catatrac device with mild cataracts is of less relevance to its intended target population.
Conclusions
This is the first study to critically assess the use of the Catatrac device as a potential screening tool for adult cataracts in the developing world. Although having a lower sensitivity for mild cataracts, it has a high specificity, sensitivity, and interobserver agreement for the significant degrees of cataracts, which are of the most relevance to its intended target population. The Catatrac device is a low-cost device that requires minimal training. This study has demonstrated the potential of this device for screening for cataract in the developing world. A larger study carried out in the developing world involving primary health care workers would be of further use in confirming the effectiveness of the Catatrac device as a cataract screening device.

References
Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004; 82 (11): 844–851.
Baltussen R, Sylla M, Mariotti SP . Cost-effectiveness analysis of cataract surgery: a global and regional analysis. Bull World Health Organ 2004; 82 (5): 338–345.
Thylefors B . A simplified methodology for the assessment of blindness and its main causes. World Health Stat Q 1987; 40 (2): 129–141.
Frick KD, Foster A . The magnitude and cost of global blindness: an increasing problem that can be alleviated. Am J Ophthalmol 2003; 135 (4): 471–476.
Zhang M, Wu J, Li L, Xu D, Lam DS, Lee J et al. Impact of cataract screening outreach in rural China. Invest Ophthalmol Vis Sci 2010; 51 (1): 110–114.
Rotchford AP, Johnson GJ . Rapid assessment of cataract surgical coverage in rural Zululand. S Afr Med J 2000; 90 (10): 1030–1032.
Wadud Z, Kuper H, Polack S, Lindfield R, Akm MR, Choudhury KA et al. Rapid assessment of avoidable blindness and needs assessment of cataract surgical services in Satkhira District, Bangladesh. Br J Ophthalmol 2006; 90 (10): 1225–1229.
Nirmalan PK, Thulasiraj RD, Maneksha V, Rahmathullah R, Ramakrishnan R, Padmavathi A et al. A population based eye survey of older adults in Tirunelveli district of south India: blindness, cataract surgery, and visual outcomes. Br J Ophthalmol 2002; 86 (5): 505–512.
Foster A . Who will operate on Africa’s 3 million curably blind people? Lancet 1991; 337 (8752): 1267–1269.
Chylack LT, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 1993; 111 (6): 831–836.
Brilliant LB, Grasset NC, Pokhrel RP, Kolstad A, Lepkowski JM, Brilliant GE et al. Associations among cataract prevalence, sunlight hours, and altitude in the Himalayas. Am J Epidemiol 1983; 118 (2): 250–264.
Chatterjee A, Milton RC, Thyle S . Prevalence and aetiology of cataract in Punjab. Br J Ophthalmol 1982; 66 (1): 35–42.
Leibowitz HM, Krueger DE, Maunder LR, Milton RC, Kini MM, Kahn HA et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973-1975. Surv Ophthalmol 1980; 24 (Suppl): 335–610.
Brilliant LB, Lepkowski JM, Musch DC . Reliability of ophthalmic diagnoses in an epidemiologic survey. Am J Epidemiol 1983; 118 (2): 265–279.
West SK, Taylor HR . The detection and grading of cataract: an epidemiologic perspective. Surv Ophthalmol 1986; 31 (3): 175–184.
Cook C, Cockburn N, van der Merwe J, Ehrlich R . Cataract and glaucoma case detection for Vision 2020 programs in Africa: an evaluation of 6 possible screening tests. J Glaucoma 2009; 18 (7): 557–562.
Ariyasu RG, Lee PP, Linton KP, LaBree LD, Azen SP, Siu AL . Sensitivity, specificity, and predictive values of screening tests for eye conditions in a clinic-based population. Ophthalmology 1996; 103 (11): 1751–1760.
Mbulaiteye SM, Reeves BC, Karabalinde A, Ruberantwari A, Mulwanyi F, Whitworth JA et al. Evaluation of E-optotypes as a screening test and the prevalence and causes of visual loss in a rural population in SW Uganda. Ophthalmic Epidemiol 2002; 9 (4): 251–262.
Sapkota YD, Pokharel GP, Nirmalan PK, Dulal S, Maharjan IM, Prakash K . Prevalence of blindness and cataract surgery in Gandaki Zone, Nepal. Br J Ophthalmol 2006; 90 (4): 411–416.
Oye JE, Kuper H . Prevalence and causes of blindness and visual impairment in Limbe urban area, South West Province, Cameroon. Br J Ophthalmol 2007; 91 (11): 1435–1439.
Brian G, Palagyi A, Ramke J, du Toit R, Naduvilath T . Cataract and its surgery in Timor-Leste. Clin Experiment Ophthalmol 2006; 34 (9): 870–879.
Oye JE, Kuper H, Dineen B, Befidi-Mengue R, Foster A . Prevalence and causes of blindness and visual impairment in Muyuka: a rural health district in South West Province, Cameroon. Br J Ophthalmol 2006; 90 (5): 538–542.
Nkomazana O . A national survey of visual impairment in Botswana. Community Eye Health 2007; 20 (61): 9.
Acknowledgements
We thank Catatrac Ltd for donating two Catatrac devices and a set of LOCS III slides for the purposes of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
We declare no financial interest in the Catatrac device or Catatrac Ltd, and have received no payment for the preparation of this manuscript.
Rights and permissions
About this article
Cite this article
Rahman, M., Rotchford, A. & Ramaesh, K. Catatrac: a novel red light-emitting diode device for screening cataracts in the developing world. Eye 27, 37–41 (2013). https://doi.org/10.1038/eye.2012.214
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2012.214