Abstract
Purpose
To compare lymphangiogenesis in primary versus recurrent pterygium.
Methods
Tissues from 88 excised primary and 34 recurrent pterygia were evaluated, and tissues from 7 nasal epibulbar conjunctivae segments were used as controls. The lymph-vascular area (LVA), lymph-microvascular density (LMD), and lymph-vascular luminal diameter (LVL) were examined and compared between the primary and recurrent pterygia. In addition, the expression of VEGF-A and VEGF-C in the primary and recurrent pterygia were determined by ELISA and real-time PCR. The relationships between the mRNA level and LVA, LMD, and LVL were clarified.
Results
Although there was no significant difference in quantification of LVL between primary and recurrent pterygia, the quantification of LVA and LMD in recurrent pterygia dramatically increased in comparison with primary pterygia (both P-values <0.01). Compared with primary pterygia, the VEGF-A and VEGF-C mRNA levels were up-regulated significantly in recurrent pterygia (both P-values <0.05). There was a significant relationship between VEGF-C mRNA and LVA, LMD, and LVL, while VEGF-A mRNA was only closely correlated with LMD in recurrent pterygia.
Conclusions
Lymphangiogenesis develops in recurrent pterygium, for which transient up-regulation of VEGF-C might be responsible.
Similar content being viewed by others
Introduction
A pterygium is a fibrovascular growth of actinically-damaged conjunctiva that extends across the limbus with invasion of the cornea. Currently, surgery is the only method for treating pterygia; however, recurrences are a major complication of the surgical treatment of pterygia. Although numerous approaches have been attempted to reduce recurrences, such as adjunctive mitomycin treatment, radiation, and amniotic membrane transplantation, recurrences still occur.1, 2 Moreover, in addition to the pathogenesis of pterygia being in question, little is known regarding differences between primary and recurrent pterygia.
Although current evidence is not definitive, immunologic mechanisms likely contribute to the development of pterygia. Pterygia samples have been shown to have increased levels of cell signaling and adhesion molecules, such as vascular cellular adhesion molecule-1 and intercellular adhesion molecule-1, and aberrant expression of human leucocyte antigen-DR.3, 4 Other signaling molecules, including E-cadherin and b-catenin, are up-regulated and concentrated in the heads of pterygia.5 Increased b-catenin has been shown to trigger certain cell cycle proteins and matrix metalloproteinases.6 An increase in mast cells, lymphocytes, plasma cells, dendritic cells, and CD4+ and CD8+ T cells in pterygia samples has also been documented.3, 4, 7 Stromal infiltrates in pterygia of T cells, with an increased helper-to-suppressor ratio, and abnormal deposits of immunoglobulins E and I have been described.8, 9 A subsequent study screened pterygial gene expression compared to normal conjunctival tissue, with a significant increase in macrophage inflammatory protein-4.10 These immunologic mechanisms have been associated with pterygia, but it is unclear if immunologic mechanisms are involved in pathogenesis or are only secondarily expressed after pterygia formation. The roles of immunologic mechanisms in pterygia formation require further elucidation.
Recently, we have shown that there is an increased lymphatic microvessel density (LMD) in pterygia.11 In addition to functioning in the maintenance of tissue fluid homeostasis, the lymphatic system also plays an essential role in the immune response to infectious agents.12, 13, 14 Whereas the blood vessels provide a route of entry for immune effector cells (eg, CD4+ alloreactive T lymphocytes and memory T lymphocytes), afferent lymphatic vessels are the exit route by which antigen-presenting cells migrate to the regional lymph nodes and lymphoid organs.15 The occurrence of lymphangiogenesis in pterygia provides further evidence that immunologic mechanisms contribute to pterygia development and growth because lymphatic vessels are regarded as an exit ‘arm’ in immunity. Moreover, whether or not immunologic mechanisms are involved in the recurrence of pterygia warrants further investigation. If such is the case, we hypothesize that there must be some differences in lymphangiogenesis between primary and recurrent pterygia.
The aims of the present study were to compare the degree of lymphangiogenesis, including lymph-vascular area (LVA), LMD, and the lymph-vascular luminal diameter (LVL), and determine the expression of VEGF-C and VEGF-A, two important factors in lymphangiogenesis, in primary and recurrent pterygia.
Materials and methods
Subjects
Eighty-eight cases of surgically-excised primary pterygia from 88 patients (39 males and 49 females; average age, 47.5±12.1 years) and 34 excised recurrent pterygia from 34 patients (15 males and 19 females average age, 53.2±17.3 years) were included in the study (Table 1). All patients underwent excision by the bare sclera technique in the Department of Ophthalmology at the Third Affiliated Hospital of Sun Yat-sen University. All of the lesions were located on the nasal side and only the heads of the pterygia were included as pterygium samples. Seven nasal epibulbar conjunctival segments, excised during cataract surgery near the limbus, were used as control tissues. Each excised tissue was divided equally into three pieces, as follows: one for immunohistochemistry; one for ELISA; and another for real-time PCR. All patients and controls were informed of the experimental nature of this procedure and signed consent was obtained beforehand. All procedures were conducted according to the principles expressed in the Declaration of Helsinki.
Immunohistochemistry
After being fixed in 10% neutral formalin for 24 h, embedded in paraffin, serially sectioned for 4 μm thickness (10 sections per sample and pterygia sections being selected from the head of pterygia to head-neck junction along the longitudinal axis), and rehydrated with graded ethanol-water mixtures, excised segments were washed with distilled water. Endogeneous peroxidase activity was blocked after incubation with 30 ml/l of hydrogen peroxidase for 20 min. Tissue sections were then autoclaved at 121°C in 10 mmol/l citrate buffer (pH 6.0) for 10 min for antigen retrieval and cooled at room temperature for 30 min. The sections were then incubated for 3 h with mouse anti-human LYVE-1 monoclonal antibody (R&D Systems, Minneapolis, MN, USA) or mouse anti-human CD31 (R&D Systems, Minneapolis, MN, USA) and biotin-marked rabbit anti-mouse immunoglobulin as the secondary antibody. Strept-avidin biotin complex (SABC)-peroxidase was used as the immune check system. The slides were visualized for peroxidase activity with diaminobenzidine (DAB), and counterstained with hematoxylin.
Quantification of immunohistochemical staining
Sections were viewed using a Zeiss Axioskop microscope and images projected to a Sony PVM1440QM video monitor using a Sony CCDIRIS video camera. Digitized images were captured using a Fujix HC-1000 3CCD high resolution color camera. Following preliminary scanning of each section at low power, 5 identified areas of high lymph-vascular density were imaged at high power (100 × ) and captured for further analysis by digital image analysis software (Axiovision 4.7.2; Carl Zeiss, Jena, Germany).
LVA quantification
Computer images were converted into a thresholded raw binary format, highlighting the immunostained lymphatic vessels with minimal highlighting of background tissue staining. These images were then analyzed using an in-house computer image analysis program that reports the proportional area of the computer image occupied by immunostained lymphatic vessels.
LMD quantification
Computer images were used to perform manual counts of stained lymph-microvessels with each vessel marked after being counted to prevent duplicate counting of vessels. Vessel counts per field were converted to vessels per mm2.
LVL quantification
Computer images were used to perform manual measurements of the maximum luminal diameter of stained microvessels for which a vessel lumen was clearly present. Each vessel was marked after luminal diameter measurement to prevent duplicate measurement of vessels.
Quantification of VEGF-A and VEGF-C proteins by ELISA
Each excised tissue was placed in 100 μl of lysis buffer (20 mM imidazole HCl, 10 mM KCl, 1 mM MgCl2, 10 mM EGTA, 1% Triton, 10 mM NaF, 1 mM Na molybdate, and 1 mM EDTA [pH 6.8]) supplemented with a protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN, USA), which was homogenized with a plastic pestle (Geno Technology, Inc., Maplewood, MO, USA) attached to a handheld drill. Tissues were homogenized in three 15-s bursts, and the suspension was incubated on ice for 10 min to allow lysis. The lysate was cleared of debris by centrifugation at 18 000 g for 15 min at 4°C and the supernatant was assayed. Total protein content was determined by a commercial assay (BCA kit; Bio-Rad, Hercules, CA, USA). Supernatant cytokine levels were determined by a sandwich enzyme-linked immunosorbent assay (ELISA) for VEGF-A and VEGF-C, according to the manufacturer’s instructions (RapidBio, Calabasas, CA, USA), and were normalized to the total protein.
RNA isolation and purification
Total RNA was isolated from the samples using TRIzol Reagent (GIBCO-BRL Life Technologies, Gaithersburg, MD, USA). RNA was prepared following the protocol from the manufacturer. The RNA pellets were washed with 75% ethanol, centrifuged, and dried. The residual DNA was removed by DNase I treatment. Pellets were resuspended in 30 μl of DEPC-treated water followed by the addition of 50 mM Tris (pH 7.5), 10 mM MgCl2, 20 U of RNase-free DNase I, and 20 U of RNase in a total volume of 60 μl. The samples were incubated at 37°C for 25 min. Then, the RNA was cleaned using an RNAeasy Mini Kit (Qiagen, Valencia, CA, USA) following the protocol provided by the manufacturer. The concentration and purity of RNA were determined by measuring optical density at 260 and 280 nm in a spectrophotometer.
Real-time reverse transcriptase PCR
cDNA was generated from the total RNA samples using a Taqman Reverse Transcription Reagents kit (Applied Biosystems, Foster City, CA, USA). To make the cDNA, the total RNA from each sample was first incubated at 25°C for 10 min, then reverse-transcribed at 48°C for 30 min. Real-time reverse transcriptase (RT)-PCR was performed using SYBR Green dye (Applied Biosystems, Foster City, CA, USA) with ABI PRISM 7900HT equipment (Applied Biosystems, Foster City, CA, USA). The primers for VEGF-A were 5′-GCAGATGTGAATGCAGACCAAA-3′(sense) and 5′-CTGCGGATCTTGGACAAACA-3′(anti-sense; GenBank number NM009505). The primers of VEGF-C were 5′-CAATTATTAGACGTTCTCTGCCAGC-3′ (sense) and 5′-GCATCGGCACATGTAGTTATTCC-3′ (anti-sense; GenBank number NM009506). DNA polymerase was first activated at 95°C for 10 min, followed by 40 cycles of denaturation for 15 s at 95°C and annealing /extension at 60°C for 1 min, according to the manufacturer’s protocol. The products were sequenced to ensure that the correct gene sequence was being amplified. All PCR reactions were performed in triplicate. Relative quantification of gene expression was carried out using the standard curve method (User Bulletin number 2, ABI PRISM 7700 Sequence Detection System). For comparison of the transcript levels between samples, standard curves were prepared for the target gene and an endogenous reference (18S ribosomal RNA). For each experimental sample, the amount of target and endogenous reference was determined from the appropriate standard curves. Then, the target amount was divided by the endogenous reference amount to obtain a normalized target value. Each of the experimental normalized sample values was divided by the normalized control sample value to generate the relative expression levels. The examinations were repeated for every sample (three times for each), then the mean values for every sample were calculated.
Statistical analysis
Analysis of the significance of differences between the two groups was performed using paired Student’s t-test (SPSS 12.0 statistical software; SPSS, Inc., Chicago, IL, USA). Pearson’s analysis was used to analyze the correlation between LVA, LMD, LVL, and VEGF-A and VEGF-C mRNA. The values were presented as the mean±SD. All reported P-values were 2-tailed, and statistical significance was defined at the α=0.05 level.
Results
Immunohistochemical staining
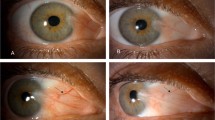
Immunohistochemistry was performed on LYVE-1 and CD31 serial sections of human pterygium tissue. Because CD31 stains blood and lymphatic vessels, and LYVE-1 stains the lymphatic endothelium,16, 17 we could identify and distinguish corneal blood and lymphatic vessels in histologic sections simultaneously. Compared with blood vessels, lymphatic vessels had a relative larger lumen and did not contain erythrocytes. Our data showed that there was a small number of CD31(+)LYVE-1(−) blood vessels, but only a few CD31(+)LYVE-1(+) lymphatic vessels in normal epibulbar conjunctivae segments. Lymphatic vessels were mildly increased in primary pterygium, but were dramatically increased in recurrent pterygium (Figure 1).
CD31 and LYVE-1 immunohistochemistry for normal human conjunctiva and pterygia. There was a small number of CD31(+)LYVE-1(−) blood vessels, but only a few CD31(+)LYVE-1(+) lymphatic vessels in normal epibulbar conjunctivae segments (a). Lymphatic vessels were moderately increased in primary pterygia (b), but were dramatically increased in recurrent pterygia (c) Footnote 1: CD31 immunohistochemistry; Footnote 2: LYVE-1 immunohistochemistry; red arrows: lymphatic vessels; blue arrows: blood vessels. Magnification for immunohistochemistry × 100).
Quantification of LVA, LMD, and LVL in primary versus recurrent pterygium
Compared with normal conjunctiva, quantification of LVA, LMD, and LVL significantly increased in primary and recurrent pterygia. Although there was no significant difference in quantification of LVL between primary and recurrent pterygia, the quantification of LVA and LMD in recurrent pterygia dramatically increased in comparison with primary pterygia (both P-values <0.01, Table 2). The increasing rate of LVA was 30%, which was in parallel with that in LMD in recurrent pterygia, suggesting lymphangiogenesis (the outgrowth of lymphatic vessels) contributed to the formation of recurrent pterygia.
Expression of VEGF-A and VEGF-C in primary versus recurrent pterygia
To elucidate and compare the role of VEGF-A and VEGF-C, we used ELISA and real-time PCR to determine the expression of VEGF-A and VEGF-C proteins and mRNA in normal conjunctiva, and primary and recurrent pterygia, respectively. Compared with normal conjunctiva, the proteins and mRNAs of VEGF-A and VEGF-C increased dramatically in primary and recurrent pterygia. Compared with primary pterygia, the increase in VEGF-A mRNA was up-regulated moderately in recurrent pterygia. However, the expression of VEGF-C mRNA was more than doubled in recurrent pterygia (Table 3). Subsequently, we compared the relationship between VEGF-A mRNA and VEGF-C mRNA in primary and recurrent pterygia, respectively. Although there was a significant relationship between VEGF-A mRNA and VEGF-C mRNA in primary pterygia, the association of them was not significant in recurrent pterygia (P>0.05, Figures 2m and n). Finally, we compared the relationship between VEGF-C mRNA and lymphangiogenesis, as well as VEGF-A mRNA and lymphangiogenesis. Our data showed that there was a significant relationship between VEGF-C mRNA and all three parameters (LVA, LMD, and LVL) in primary and recurrent pterygium (Figures 2d–f and j–l)., while VEGF-A mRNA was closely correlated with LVA and LMD in primary pterygium but only LMD in recurrent pterygium (Figures 2a–c and g–i).
The relationship between VEGF-A mRNA, VEGF-C mRNA, and lymphangiogenesis in primary vs recurrent pterygia. The relationships were significant between VEGF-A mRNA and lymph-vascular area, and VEGF-A mRNA and lymph-microvascular density, but the relationship between VEGF-A mRNA and lymph-vascular luminal diameter was not significant in patients with primary pterygia (a–c). There was also a significant relationship between VEGF-A mRNA and lymph-microvascular density in recurrent pterygia, but the relationships between VEGF-A mRNA and lymph-vascular area, and between VEGF-A mRNA and lymph-vascular luminal diameter, were not significant (g–i). The levels of VEGF-C mRNA correlated closely with all three parameters (lymph-vascular area, lymph-microvascular density, and lymph-vascular luminal diameter) in both primary pterygia (d–f) and recurrent pterygia (j–l). Although there was a significant relationship between VEGF-A mRNA and VEGF-C mRNA in primary pterygia (m) (r=0.67, P<0.01), the relationship between VEGF-A mRNA and VEGF-C mRNA was not significant in recurrent pterygia (n) ((r=0.17, P>0.05).
Discussion
In our previous study we showed that lymphangiogenesis occurred in primary pterygium.11 Recently, Cimpean et al examined human pterygium using immunohistochemistry and reported increased lymphatic microvessel density was observed in the human pterygium compared to normal conjunctiva.18 Moreover, they also found that D2-40-positive lymphatic endothelial cells were actively proliferating, as assessed by Ki-67 immunostaining, while in normal conjunctiva proliferating lymphatic endothelial cells were not detectable. These data clearly indicate the presence of active proliferating lymphatic vessels in human pterygium, suggesting that active lymphangiogenesis occurs in this pathologic condition. Recent research involving corneal lymphangiogenesis has demonstrated that afferent corneal lymphatics may be equal, or even more important than efferent corneal blood vessels in corneal immunity.19, 20, 21 The presence of an increased LMD in primary pterygium provides evidence that immunologic mechanisms play a role in the development of pterygium.
However, compared with reports on primary pterygium, there are few studies that make a distinction between primary and recurrent pterygia. Whether or not immunologic mechanisms contribute to the recurrence of pterygia remain unknown. To elucidate the difference in lymphatic vessels between primary and recurrent pterygia, we conducted a comparative evaluation of lymphangiogenesis using three parameters (LVA, LMD, and LVL). Our analyses showed a greater increase in LVA and LMD in recurrent pterygia in comparison with that in primary pterygia, suggesting that there is a significant outgrowth and hyperplasia of lymphatic vessels in recurrent pterygium. Subsequently, we examined the increasing rates of LVA, LMD, and LVL in recurrent pterygia. We found that although there was no significant difference in LVL between primary and recurrent pterygia, the rates of LVA and LMD increased to 30%, thus there were many new lymphatic vessel formation during the recurrence of pterygium. On the basis of above, we have documented for the first time that lymphangiogenesis occurs and develops in recurrent pterygium. Therefore, strategies of anti-lymphangiogenic therapy should be further investigated to improve the prognosis of pterygium.
Recently, Kajiya et al demonstrated that acute UVB irradiation of the skin induces lymphatic vessel enlargement that is associated with the expression of the potent lymphangiogenic factor, VEGF-C.22 Although the pathogenesis has not been completely understood, exposure to UVB irradiation is believed to be the leading cause of primary pterygium.23, 24 However, Kajiya et al showed that acute UVB irradiation resulted in down-regulation of VEGF-C expression and the density of lymphatic vessels had no significant difference between non-irradiated skin and UVB-irradiated skin, which was contradictory to our results.22 We believe that such a difference is due to the role of lymphatic vessels in the response to acute or chronic UVB irradiation. Because pterygium is an ocular surface disease of humans attributed to chronic UVB exposure, it is not surprising that lymphangiogenesis develops in pterygium. In fact, our results are consistent with a similar experiment, which was also conducted by Kajiya et al, which showed a prominent enlargement and an increasing number of lymphatic vessels of murine skin after chronic UVB irradiation. However, Kajiya et al argued that the levels of expression of VEGF-A, but not of the known lymphangiogenesis factor, VEGF-C, are responsible for lymphangiogenesis in such a chronic UVB-irradiated injury.25 Considering that other studies have also shown that VEGF-A plays a more important role in lymphangiogenesis,26, 27 we examined the expression of VEGF-C and VEGF-A, and compared the levels of expression with LVA, LVD, and LVL in pterygium.
In an earlier study, Cursiefen et al28 reported that VEGF-A–activated inflammatory macrophages released VEGF-C/-D that contributed to lymphangiogenesis in inflammatory corneas, which suggested an indirect role was present in VEGF-A induced lymphangiogenesis. We found VEGF-A was closely related with VEGF-C in primary pterygia, and VEGF-A correlated closely with LVA and LVD. Therefore, the possibility of the indirect lymphangiogenesis of VEGF-A via the high VEGF-C expression in primary pterygia could not be excluded. However, the significant correlation between VEGF-A and VEGF-C in recurrent pterygia was not found, while VEGF-A correlated closely with LVD. It cbviously could not be explained by such a indirect role of VEGF-A induced lymphangiogenesis. Recently, there is convincing evidence that VEGF-A can be lymphangiogenic via VEGF-A receptors for VEGFR2, which are also expressed on lymphatic endothelial cells.29, 30 This possibility might be partially explained by the close relationship between VEGF-A mRNA and lymphangiogenesis in pterygium, especially in recurrent pterygium. In addition to VEGFR2, VEGF-C also binds to VEGFR3, which has been shown to be essential for the formation of lymphatic vessels.31, 32 Because the increasing rates of VEGF-C are much higher than VEGF-A, such a VEGF-C-VEGFR3 pathway might play a more important role in lymphangiogenesis in recurrent pterygia.

Accession codes
References
Di Girolamo N, Chui J, Coroneo MT, Wakefield D . Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res 2004; 23: 195–228.
Kuo CH, Miyazaki D, Yakura K, Araki-Sasaki K, Inoue Y . Role of periostin and interleukin-4 in recurrence of pterygia. Invest Ophthalmol Vis Sci 2010; 51: 139–143.
Beden U, Irkec M, Orhan D, Orhan M . The roles of T-lymphocyte subpopulations (CD4 and CD8), intercellular adhesion molecule-1 (ICAM-1), HLA-DR receptor, and mast cells in etiopathogenesis of pterygium. Ocul Immunol Inflamm 2003; 11: 115–122.
Ioachim-Velogianni E, Tsironi E, Agnantis N, Datseris G, Psilas K . HLA-DR expression in pterygium epithelial cells and lymphocyte subpopulations: an immunohistochemistry study. Ger J Ophthalmol 1995; 4: 123–129.
Kase S, Osaki M, Sato I, Takahashi S, Nakanishi K, Yoshida K et al. Immunolocalisation of E-cadherin and beta-catenin in human pterygium. Br J Ophthalmol 2007; 91: 1209–1212.
Kato N, Shimmura S, Kawakita T, Miyashita H, Ogawa Y, Yoshida S et al. Beta-catenin activation and epithelialmesenchymal transition in the pathogenesis of pterygium. Invest Ophthalmol Vis Sci 2007; 48: 1511–1517.
Ribatti D, Nico B, Maxia C, Longo V, Murtas D, Mangieri D et al. Neovascularization and mast cells with tryptase activity increase simultaneously in human pterygium. J Cell Mol Med 2007; 11: 585–589.
Pinkerton OD, Hokama Y, Shigemura LA . Immunologic basis for the pathogenesis of pterygium. Am J Ophthalmol 1984; 98: 225–228.
Liu L, Yang D . Immunological studies on the pathogenesis of pterygium. Chin Med Sci J 1993; 8: 84–88.
John-Aryankalayil M, Dushku N, Jaworski CJ, Cox CA, Schultz G, Smith JA et al. Microarray and protein analysis of human pterygium. Mol Vis 2006; 12: 55–64.
Ling S, Liang L, Lin H, Li W, Xu J . Increasing lymphatic microvessel density in primary pterygia. Arch Ophthalmol 2012; 130: 735–742.
Patel SP, Dana R . Corneal lymphangiogenesis: implications in immunity. Semin Ophthalmol 2009; 24: 135–138 Review.
Cueni LN, Detmar M . The lymphatic system in health and disease. Lymphat Res Biol 2008; 6: 109–122 Review.
Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest 2005; 115: 2363–2372.
Cursiefen C, Chen L, Dana MR, Streilein JW . Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea 2003; 22: 273–281 Review.
Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell 2004; 6: 333–345.
Bjorndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y et al. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci USA 2005; 102: 15593–15598.
Cimpean AM, Poenaru Sava M, Raica M, Ribatti D . Preliminary evidence of the presence of lymphatic vessels immunoreactive for D2-40 and Prox-1 in human pterygium. Oncol Rep 2011; 26: 1111–1113.
Ling S, Qi C, Li W, Xu J, Kuang W . Crucial role of corneal lymphangiogenesis for allograft rejection in alkali-burned cornea bed. Clin Experiment Ophthalmol 2009; 37: 874–883.
Dietrich T, Bock F, Yuen D, Hos D, Bachmann BO, Zahn G et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol 2010; 184: 535–539.
Chen L, Hamrah P, Cursiefen C, Zhang Q, Pytowski B, Streilein JW et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med 2004; 10: 813–815.
Kajiya K, Sawane M, Huggenberger R, Detmar M . Activation of the VEGFR-3 pathway by VEGF-C attenuates UVB-induced edema formation and skin inflammation by promoting lymphangiogenesis. J Invest Dermatol 2009; 129: 1292–1298.
Nolan TM, DiGirolamo N, Sachdev NH, Hampartzoumian T, Coroneo MT, Wakefield D . The role of ultraviolet irradiation and heparin-binding epidermal growth factor-like growth factor in the pathogenesis of pterygium. Am J Pathol 2003; 162: 567–574.
Di Girolamo N, Kumar RK, Coroneo MT, Wakefield D . UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci 2002; 43: 3430–3437.
Kajiya K, Hirakawa S, Detmar M . Vascular endothelial growth factor-A mediates ultraviolet B-induced impairment of lymphatic vessel function. Am J Pathol 2006; 169: 1496–1503.
Wuest TR, Carr DJ . VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med 2010; 207: 101–115.
Bachmann BO, Luetjen-Drecoll E, Bock F, Wiegand SJ, Hos D, Dana R et al. Transient postoperative vascular endothelial growth factor (VEGF)-neutralisation improves graft survival in corneas with partly regressed inflammatory neovascularisation. Br J Ophthalmol 2009; 93: 1075–1080.
Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 2004; 113: 1040–1050.
Pavlakovic H, Becker J, Albuquerque R, Wilting J, Ambati J . Soluble VEGFR-2: an antilymphangiogenic variant of VEGF receptors. Ann NY Acad Sci 2010; 1207 (Suppl 1): E7–E15.
Becker J, Pavlakovic H, Ludewig F, Wilting F, Weich HA, Albuquerque R et al. Neuroblastoma progression correlates with downregulation of the lymphangiogenesis inhibitor sVEGFR-2. Clin Cancer Res 2010; 16: 1431–1441.
Bock F, Onderka J, Dietrich T, Bachmann B, Pytowski B, Cursiefen C . Blockade of VEGFR3-signalling specifically inhibits lymphangiogenesis in inflammatory corneal neovascularisation. Graefes Arch Clin Exp Ophthalmol 2008; 246: 115–119.
Chen L, Hamrah P, Cursiefen C, Zhang Q, Pytowski B, Streilein JW et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Ocul Immunol Inflamm 2007; 15: 275–278.
Acknowledgements
This study was supported by China Natural Science Foundation (81070711), and Guangdong Natural Science Foundation (S2011010006061) and Guangdong Provincial Science and technology projects (2010B060200008). We thank Dr Chaoyang Li and Dr Wenhui Kuang for their invaluable technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ling, S., Li, Q., Lin, H. et al. Comparative evaluation of lymphatic vessels in primary versus recurrent pterygium. Eye 26, 1451–1458 (2012). https://doi.org/10.1038/eye.2012.194
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2012.194
Keywords
This article is cited by
-
Management of primary pterygium with intra-lesional injection of 5 flurouracil and bevacizumab (Avastin)
Eye (2019)
-
UV light-blocking contact lenses protect against short-term UVB-induced limbal stem cell niche damage and inflammation
Scientific Reports (2018)
-
A novel graft option after pterygium excision: platelet-rich fibrin for conjunctivoplasty
Eye (2017)