Abstract
Purpose
Retinal pigment epithelium (RPE) tears may develop as a complication after anti-VEGF (vascular endothelial growth factor) treatment for pigment epithelial detachments (PEDs) in exudative age-related macular degeneration (AMD). This retrospective study analyses best-corrected visual acuity (BCVA) and foveal involvement after RPE tears that are associated with anti-VEGF therapy due to PED in exudative AMD.
Methods
A total of 37 patients with RPE tears during anti-VEGF therapy (bevacizumab 12, ranibizumab 21 and pegaptanib 4 eyes) for progressive PED in AMD (PED with occult choroidal neovascularization 25 eyes and PED with retinal angiomatous proliferation 12 eyes) were included in this study. We analyzed BCVA and different morphologic aspects by means of appearance on fluorescein angiography and optical coherence tomography. Mean follow-up was 88 weeks.
Results
RPE tears were diagnosed a mean of 56 days after the first injection. BCVA deteriorated after RPE tear and during follow-up significantly (P<0.001), with 53.2% of eyes being legally blind (WHO, world health organization) at 12 months. RPE-free foveal area, foveal wrinkling of the RPE, and fibrotic scar development were significantly associated with worse visual acuity.
Discussion
RPE tears can be observed in 12–15% of treated eyes during anti-VEGF therapy for PED in exudative AMD. Owing to the close time relationship with the therapy, this complication must be taken into consideration. Visual prognosis is associated with a decrease in vision in the long term, often resulting in a severe visual disability. Relevant factors for a negative visual prognosis were the potential foveal involvement of the central RPE and morphologic fibrovascular transformation of the RPE tear.
Similar content being viewed by others
Introduction
Tears of the retinal pigment epithelium (RPE) are known to develop in eyes affected by exudative age-related macular degeneration (AMD). In most cases, the RPE tear is part of the natural history of pigment epithelial detachment (PED) that has developed as a result of occult choroidal neovascularization, retinal angiomatous proliferation, or polypoidal choroidal vasculopathy.1, 2, 3 Such tears were first described as a spontaneous complication of PEDs in AMD by Hoskin et al.4 During follow-up, a RPE tear develops in ∼10% of eyes in which this type of exudative AMD has developed.5
RPE tears also represent a complication that develops in association with various treatments for exudative AMD, such as laser photocoagulation,6, 7 transpupillary thermotherapy,8 and photodynamic therapy (PDT).9, 10, 11, 12, 13 Owing to the fact that such conditions were often associated with considerable subretinal bleeding and the development of disciform scars, the visual course of eyes with RPE tears was in general devastating14 and no treatment was possible.
In addition, RPE tears that have developed in eyes with PED after anti-VEGF (vascular endothelial growth factor) treatment have been reported to have a minor effect on the short-term visual course.15, 16, 17, 18, 19, 20, 21, 22 However, little is known about the long-term outcome in these patients. The aim of the present study, therefore, was to evaluate the long-term visual and morphologic prognosis after RPE tears in association with anti-VEGF treatment.
Materials and methods
The clinical course of 37 patients (29 female, 8 male; mean age 78.8 (63–90 years)) with new RPE tears during repeated anti-VEGF therapy (31 eyes ranibizumab, 12 eyes bevacizumab, and 4 eyes pegaptanib) for progressive PED (increasing PED or visual loss) was followed over a mean of 88 weeks (SD±51 weeks). PEDs were associated with occult choroidal neovascularization in 67.6% (25/37) and with retinal angiomatous proliferation lesions in 32.4% (12/37) of the eyes.
At baseline examination, the presence of PED and neovascularization was confirmed by fluorescein angiography and optical coherence tomography (OCT) imaging (Stratus-OCT Zeiss, Software 4.0, Jena, Germany). Choroidal neovascularization lesions and associated components were classified according to the recommendations of the Macular Photocoagulation Study Group.23, 24 Treatment of the PED was recommended if progression of the disease, defined by an increase in sub-RPE fluid, sub- or intraretinal fluid and deterioration of best-corrected visual acuity (BCVA), was observed at 3-monthly reexaminations. Intravitreal anti-VEGF therapy was given in accordance with the recommendations of the German Ophthalmologic Association (DOG).25 Intravitreal injections consisted of either 1.25 mg/0.05 ml bevacizumab, 0.5 mg/0.05 ml ranibizumab, or 0.3 mg/0.09 ml pegaptanib. All bevacizumab preparations were obtained from a qualified pharmacy. No intraoperative complications were observed in any of the patients.
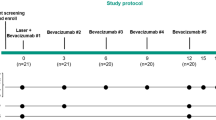
The initial treatment consisted of 3-monthly injections in all patients. The follow-up examinations were scheduled after 1, 3, 6, and 12 months, or more often if any signs of new visual symptoms developed, and included BCVA, ophthalmoscopy, fluorescein angiography, and OCT in order to evaluate the functional and morphological changes after treatment. Retreatment was recommended if during follow-up a visual function decreased further and was associated with new or increased sub- or intraretinal fluid. As PED only partially regressed in most patients, this characteristic was not an indication for retreatment.
During follow-up all patients developed a RPE tear in the area of the PED. The time at which the RPE tear developed in relation to the anti-VEGF therapy and its influence on visual function were recorded, too. Therefore, the patients were assigned to different visual groups at every follow-up visit: eyes with reading ability and BCVA<logMAR (logarithm of minimum angle of resolution) 0.5; low-vision group with BCVA logMAR0.5–logMAR 1.0; WHO (world health organization) legal blindness group with BCVA>logMAR 1.0 but <logMAR 1.5; and legal blindness (Germany) group with BCVA>logMAR 1.5. In order to evaluate relevant morphologic factors for the visual prognosis, the visual course was analyzed with respect to several morphologic characteristics: absence of the RPE within a radius of 750 μm around the foveola; wrinkling of the RPE within a radius of 750 μm around the foveola; development of either subretinal fibrosis, hard exudates and retinal cystoid alteration, or an atrophic scar.
The results are presented using descriptive statistics (including means, one-way analysis of variance), χ2-test and the repeated measures analysis of variance calculated by using MedCalc software Version 11.3.0.0 (Medcalc Software, Mariakerke, Belgium).
Results
The development of a RPE tear during anti-VEGF therapy could be observed within 11 days to 46.3 weeks after the initial injection (median 56 days; SD±56 days from injection to tear; Figure 1). No significant change in visual acuity at the time the RPE tear developed was observed in most patients, but during follow-up a significant linear decrease in BCVA was revealed by repeated measures analysis of variance (ɛ=0.77, P<0.001/Plinear<0.001; Figure 2). This constant decrease in visual function resulted in severe visual disability in many eyes, with only 15.6% of eyes having reading ability, but 31.2% of eyes showing low vision, 21.9% of eyes being legally blind (WHO), and 31.2% of eyes being legally blind (Germany) 12 months after RPE tear appearance (Figure 3).
The morphologic analysis of RPE tears revealed a RPE-free zone within a radius of 750 μm around the fovea in 19 patients (51.4%). Differentiation according to this RPE-free zone predicted a significantly different visual course (ɛ=0.8, P=0.02; Figure 4). In contrast, wrinkling of the RPE within a radius of 750 μm around the foveola alone, which was observed in 28 eyes (75.7%), only had a marginal influence on the visual course (ɛ=0.77, P=0.07, repeated measures analysis of variance), while development of a disciform scar was significantly associated with a worse visual prognosis in comparison with eyes in which an atrophic area developed (P=0.04; Figure 5).
Discussion
The development of RPE tears during anti-VEGF therapy for PED in exudative AMD has been observed in about 12–17% of treated eyes.20, 26, 27 This incidence is similar to that reported for the natural history of untreated PED.4, 5 However, in both the published literature and the present study these RPE tears always developed relatively close to the time when the anti-VEGF therapy was started (mostly 1 (Wong et al28) to 2 months20, 27). Thus, morphologic processes might be initiated during anti-VEGF therapy that result either in tractional forces at the level of the RPE, as has been suggested as the underlying cause of RPE tear development after laser coagulation, or result in tangential stress because of the increasing volume of the PED, as has been suggested for PDT. Certainly, the time association between the development of RPE tears and the begin of anti-VEGF therapy cannot prove a causative relation, but this should still be taken into consideration as a possible complication when recommending anti-VEGF therapy to patients with PED in AMD.
In previously published patient series, most often only a short follow-up period was available.15, 16, 17, 18, 19, 20, 21, 22 As the acute visual consequences are only limited after a RPE tear has been initiated, a good visual prognosis was often presumed for this complication, which is in agreement with our findings. However, with longer follow-up this prognosis decreased significantly, with a mean loss in BCVA deterioration.29 The development of a RPE tear might not always affect vision in an individual patient,20, 21, 22 but as demonstrated in other larger case series30 and also our study, the majority will experience progressive visual loss during longer-term follow-up. Not only a statistically significant deterioration in mean BCVA was observed during the follow-up compared with BCVA before injection (P<0.001), but also a devastating deterioration in visual ability after 12 months was present, with nearly one-third of all patients being legally blind (Germany) with a BCVA of logMAR 1.5 or worse.
One major factor for the poorer visual prognosis in specific subgroups might be the initial foveal involvement in the contraction of the RPE, resulting in a RPE-free central retina. Only the changes in morphology of the RPE (defined as wrinkling of the central RPE as in our study) were not associated with a significantly worse prognosis. The negative effect of a central, RPE-free area on the visual prognosis has been speculated in previous studies17, 20, 21, 28, 31 and would be consistent with the results of our investigation. Other studies could even demonstrate a gradual decrease in visual prognosis with increasing RPE contraction, and associated this with a worse classification of RPE tear severity.30 The frequency of foveal involvement can range from 23% (Chan et al20) to 36% (Kook et al21) or even 75% (Gamulescu et al17). This wide range may be due to the different definitions for foveal involvement in these studies, but the same general trend could be seen in all reports. As a relevant factor for identifying a PED with high risk for the development of RPE tear with foveal involvement, size and height of the initial PED has been suggested.20, 27 To further investigate relevant factors of PED to develop RPE tears, new insights into the morphology of PED by spectral domain optical coherence tomography may be possible. Not only the size and specific location of the associated neovascularization, but also the intensity of the proposed hydrophobic barrier in Bruch's membrane may have an important role. Also, the integrity and ‘health’ of the RPE as demonstrated on autofluorescence images should be considered as relevant, and investigated in future studies. But because neither the risk of developing an RPE tear nor the possible foveal involvement can presently be influenced by the patient or treating doctor, the only therapeutic option to be considered in second eye involvement and reduced visual acuity is macular surgery.
An additional prognostic factor, especially for the long-term visual result, is the individual but quite different morphologic courses of the disease after the development of RPE tears. Previously, the spontaneous development of RPE tears was associated with massive fibrovascular scarring.32 Such a severe disciform fibrovascular transformation was also visible during long-term follow-up in 29.7% of the patients, while others (70.3%) develop atrophy in the RPE-free area. The former clinical course was associated with a much worse visual prognosis than the atrophic form in our study. Relevant factors to distinguish between the two groups with this major and visually important different morphologic course have not been identified so far. A hope to minimize this fibrovascular transformation might be a more proactive OCT-based retreatment strategy and a long ongoing anti-VEGF therapy. However, a positive long-term effect of such a strategy is still to be proven.
In conclusion, the development of RPE tears can be observed in 12–17% of treated eyes during anti-VEGF therapy for PED in exudative AMD. Owing to the close time relationship with the therapy, this complication must be taken into consideration and explained to the patient. Even if the short-term visual prognosis is relatively unchanged by RPE tears, visual prognosis is associated with a slow decrease in vision in the long term, often resulting in a severe visual disability. One relevant factor for a negative visual prognosis was the potential foveal involvement of the central RPE. As the extent of the RPE tear cannot be influenced, the only therapeutic option to be considered is macular surgery. Morphologic fibrovascular transformation of the RPE tear represents another important factor. Whether this morphologic development can be altered into the more benign, atrophic form of RPE tear by adopting more proactive retreatment strategies should be investigated in future studies.

References
Michels S, Aue A, Simader C, Geitzenauer W, Sacu S, Schmidt-Erfurth U . Retinal pigment epithelium tears following verteporfin therapy combined with intravitreal triamcinolone. Am J Ophthalmol 2006; 141 (2): 396–398.
Muller C, Spital G, Radermacher M, Dohrmann J, Lommatzsch A, Pauleikhoff D . Pigment epithelium detachments in AMD (age-associated macular degeneration) and ‘polypoid choroidal vasculopathy’. A fluorescein and indocyanine green angiography study. Ophthalmologe 2002; 99 (2): 85–89.
Panagiotidis D, Karagiannis DA, Baltatzis S . Photodynamic therapy in retinal angiomatous proliferation stage I. Eur J Ophthalmol 2006; 16 (2): 326–329.
Hoskin A, Bird AC, Sehmi K . Tears of detached retinal pigment epithelium. Br J Ophthalmol 1981; 65 (6): 417–422.
Pauleikhoff D, Loffert D, Spital G, Radermacher M, Dohrmann J, Lommatzsch A et al. Pigment epithelial detachment in the elderly. Clinical differentiation, natural course and pathogenetic implications. Graefes Arch Clin Exp Ophthalmol 2002; 240 (7): 533–538.
Gass JD . Retinal pigment epithelial rip during krypton red laser photocoagulation. Am J Ophthalmol 1984; 98 (6): 700–706.
Yeo JH, Marcus S, Murphy RP . Retinal pigment epithelial tears. Patterns and prognosis. Ophthalmology 1988; 95 (1): 8–13.
Thompson JT . Retinal pigment epithelial tear after transpupillary thermotherapy for choroidal neovascularization. Am J Ophthalmol 2001; 131 (5): 662–664.
Arnold JJ, Blinder KJ, Bressler NM, Bressler SB, Burdan A, Haynes L et al. Acute severe visual acuity decrease after photodynamic therapy with verteporfin: case reports from randomized clinical trials-TAP and VIP report no. 3. Am J Ophthalmol 2004; 137 (4): 683–696.
Axer-Siegel R, Ehrlich R, Rosenblatt I, Kramer M, Priel E, Yassur Y et al. Photodynamic therapy for occult choroidal neovascularization with pigment epithelium detachment in age-related macular degeneration. Arch Ophthalmol 2004; 122 (4): 453–459.
Gelisken F, Inhoffen W, Partsch M, Schneider U, Kreissig I . Retinal pigment epithelial tear after photodynamic therapy for choroidal neovascularization. Am J Ophthalmol 2001; 131 (4): 518–520.
Goldstein M, Heilweil G, Barak A, Loewenstein A . Retinal pigment epithelial tear following photodynamic therapy for choroidal neovascularization secondary to AMD. Eye 2005; 19 (12): 1315–1324.
Pece A, Introini U, Bottoni F, Brancato R . Acute retinal pigment epithelial tear after photodynamic therapy. Retina 2001; 21 (6): 661–665.
Schoeppner G, Chuang EL, Bird AC . The risk of fellow eye visual loss with unilateral retinal pigment epithelial tears. Am J Ophthalmol 1989; 108 (6): 683–685.
Carvounis PE, Kopel AC, Benz MS . Retinal pigment epithelium tears following ranibizumab for exudative age-related macular degeneration. Am J Ophthalmol 2007; 143 (3): 504–505.
Dhalla MS, Blinder KJ, Tewari A, Hariprasad SM, Apte RS . Retinal pigment epithelial tear following intravitreal pegaptanib sodium. Am J Ophthalmol 2006; 141 (4): 752–754.
Gamulescu MA, Framme C, Sachs H . RPE-rip after intravitreal bevacizumab (Avastin) treatment for vascularised PED secondary to AMD. Graefes Arch Clin Exp Ophthalmol 2007; 245 (7): 1037–1040.
Lee GK, Lai TY, Chan WM, Lam DS . Retinal pigment epithelial tear following intravitreal ranibizumab injections for neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2007; 245 (8): 1225–1227.
Spandau UH, Jonas JB . Retinal pigment epithelium tear after intravitreal bevacizumab for exudative age-related macular degeneration. Am J Ophthalmol 2006; 142 (6): 1068–1070.
Chan CK, Meyer CH, Gross JG, Abraham P, Nuthi AS, Kokame GT et al. Retinal pigment epithelial tears after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Retina 2007; 27 (5): 541–551.
Kook D, Wolf A, Neubauer AS, Haritoglou C, Priglinger SG, Kampik A et al. Retinal pigment epithelial tears after intravitreal injection of bevacizumab for AMD. Frequency and progress. Ophthalmologe 2008; 105 (2): 158–164.
Smith BT, Kraus CL, Apte RS . Retinal pigment epithelial tears in ranibizumab-treated eyes. Retina 2009; 29 (3): 335–339.
Group MPS . Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study. Arch Ophthalmol 1991; 109 (9): 1242–1257.
Participants VR . Guidelines for using verteporfin (Visudyne) in photodynamic therapy for choroidal neovascularization due to age-related macular degeneration and other causes: update. Retina 2005; 25 (2): 119–134.
Jaissle GB, Szurman P, Bartz-Schmidt KU . Recommendation for the implementation of intravitreal injections—statement of the German Retina Society, the German Society of Ophthalmology (DOG) and the German Professional Association of Ophthalmologists (BVA). Klin Monbl Augenheilkd 2005; 222 (5): 390–395.
Lommatzsch A, Heimes B, Gutfleisch M, Spital G, Zeimer M, Pauleikhoff D . Serous pigment epithelial detachment in age-related macular degeneration: comparison of different treatments. Eye (Lond) 2009; 23 (12): 2163–2168.
Chiang A, Chang LK, Yu F, Sarraf D . Predictors of anti-VEGF-associated retinal pigment epithelial tear using FA and OCT analysis. Retina 2008; 28 (9): 1265–1269.
Wong LJ, Desai RU, Jain A, Feliciano D, Moshfeghi DM, Sanislo SR et al. Surveillance for potential adverse events associated with the use of intravitreal bevacizumab for retinal and choroidal vascular disease. Retina 2008; 28 (8): 1151–1158.
Kiss C, Michels S, Prager F, Geitzenauer W, Schmidt-Erfurth U . Retinal pigment epithelium tears following intravitreal ranibizumab therapy. Acta Ophthalmol Scand 2007; 85 (8): 902–903.
Sarraf D, Reddy S, Chiang A, Yu F, Jain A . A new grading system for retinal pigment epithelial tears. Retina 2010; 30 (7): 1039–1045.
Shima C, Sakaguchi H, Gomi F, Kamei M, Ikuno Y, Oshima Y et al. Complications in patients after intravitreal injection of bevacizumab. Acta Ophthalmol Scand 2008; 86 (4): 372–376.
Chuang EL, Bird AC . Repair after tears of the retinal pigment epithelium. Eye 1988; 2 (Part 1): 106–113.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gutfleisch, M., Heimes, B., Schumacher, M. et al. Long-term visual outcome of pigment epithelial tears in association with anti-VEGF therapy of pigment epithelial detachment in AMD. Eye 25, 1181–1186 (2011). https://doi.org/10.1038/eye.2011.146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2011.146
Keywords
This article is cited by
-
Predicting retinal pigment epithelium remodelling and its functional impact
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Retinal pigment epithelium apertures as a late complication of longstanding serous pigment epithelium detachments in chronic central serous chorioretinopathy
Eye (2019)
-
Atypical retinal pigment epithelial defects with retained photoreceptor layers: a so far disregarded finding in age related macular degeneration
BMC Ophthalmology (2017)
-
OCT angiography documented reperfusion of translocated autologous full thickness RPE-choroid graft for complicated neovascular age-related macular degeneration
Eye (2017)
-
Change in vision after retinal pigment epithelium tear following the use of anti-VEGF therapy for age-related macular degeneration
Graefe's Archive for Clinical and Experimental Ophthalmology (2016)