Abstract
Purpose
To study visual and anatomical outcomes of sequenced combined therapy using intravitreal bevacizumab followed by photodynamic therapy (PDT) in eyes with retinal angiomatous proliferation (RAP). Safety and rate of intravitreal injections were also evaluated.
Methods
We conducted a prospective non-comparative pilot study of consecutive patients newly diagnosed with RAP. PDT guided by indocyanine green (ICG) angiography was applied 8±2 days after the intravitreal bevacizumab (1.25 mg) injection. At baseline and every month after the injection, best-corrected visual acuity (BCVA) measurement, complete eye examination including dynamic fluorescein and ICG angiography, and optical coherence tomography (OCT) were performed.
Results
In all, 21 eyes of 18 patients with RAP were enrolled. The mean age was 77 (range 65–86) years. Mean visual acuity at baseline was 0.63±0.25 logMAR. After treatment BCVA showed no statistically significant differences between each visit (P=0.10, ANOVA). At 9 months, the BCVA improved by three or more lines in three eyes (14%), remained stable in twelve eyes (57%), and worsened in six eyes (29%). Foveal thickness decreased significantly between baseline and all the follow-up visits (P<0.01, ANOVA). A total of 36 intravitreal injections were given during the study with a mean of 1.7 injections per eye (range 1–3 injections per eye). No ocular or systemic adverse events were reported.
Conclusion
A possible synergistic effect may arise from the combination of intravitreal bevacizumab with PDT for the treatment of RAP. These findings also suggest a possible benefit of combo therapy in the rate of intravitreal re-injections.
Similar content being viewed by others
Introduction
Retinal angiomatous proliferation (RAP) has recently been described as a well-distinct form of neovascular age-related macular degeneration (ARMD) with poor functional prognosis.1 It is estimated to represent ∼10–15% of patients newly diagnosed with neovascular ARMD. On the basis of the presumed origin of the neovascular process in the retina, two different classifications have been proposed to characterize the clinical manifestations and progressive vasogenic changes in this entity.2, 3 Whatever the initial location might be, advances in fundus imaging have stressed the importance of differentiating RAP from Type 1 or Type 2 neovascularization in ARMD. Freund et al4 have recently proposed the more descriptive term ‘Type 3’ for this entity to distinguish and emphasize the intraretinal location of the vascular complex regardless of its origin.
Treatment of this form of ARMD has been disappointing so far.5, 6, 7, 8, 9, 10 Recently, promising results with sequential combined treatment with intravitreal triamcinolone and photodynamic therapy (PDT) were reported, but complications were common.11, 12 Only few uncontrolled studies reporting short-term experience with intravitreal injection of anti-vascular endothelial growth factor (VEGF) in RAP are available in the literature. They show favourable outcomes but frequent intravitreal injections are expected.13, 14, 15, 16
A combination therapy of bevacizumab and PDT has been hypothesized as being promising for neovascular ARMD, especially by prolonging the intervals between repeated injections.17 Recent reports seem to confirm that combined intravitreal bevacizumab and PDT effectively maintained or improved visual acuity also for RAP lesions.18, 19
The aim of this study was to determine visual and anatomical outcomes after intravitreal injection of the VEGF antibody bevacizumab followed by PDT in RAP lesions. Safety and number of required intravitreal re-injections were also evaluated.
Methods
We conducted a prospective pilot study of consecutive patients newly diagnosed with RAP in ARMD treated with sequenced combined intravitreal injection of bevacizumab (Avastin, Roche, Basel, Switzerland) and PDT with verteporfin (Visudyne, Novartis, Basel, Switzerland).
The study was performed in accordance with ethical standards of the Declaration of Helsinki. Informed consent, particularly with regard to the off-label use and the potential for recognized side effects associated with intravenous administration of bevacizumab, was obtained from all patients.
Patients presenting other ocular conditions leading to neovascularization (pathological myopia or inflammatory disease) or compromising vision in the study eye were excluded from the study.
At each monthly visit, each patient underwent best-corrected visual acuity (BCVA) measurement, complete eye examination including digital dynamic fluorescein and indocyanine green (ICG) angiography using a confocal scanning laser ophthalmoscope (Heidelberg Retina Angiograph, Heidelberg Engineering, Heidelberg, Germany) and optical coherence tomography (OCT) (Stratus OCT scanner with version 4.0.2 software, Carl Zeiss Meditec GmbH, Oberkochen, Germany).
BCVA was evaluated and expressed in logMAR using ETDRS charts at 4- and 1-m distance. Decrease in visual acuity was considered significant with a loss of three or more lines. Improvement of visual acuity was considered as a gain of three or more lines. Any outcome in between was considered to be a stabilization of visual acuity.
For each patient, OCT examination was performed using the Fast Macular Thickness acquisition protocol and the retinal map analysis protocol.
For statistical analysis we considered the following parameters:
-
Foveal thickness (defined as field 1 value)
-
3-mm thickness (defined as field 1–5 mean value)
-
6-mm thickness (defined as field 1–9 mean value)
-
Max thickness (defined as the highest field value among the nine fields)
-
Volume (given by the software)
The nine OCT zones were called ETDRS-type regions as already described before.20
A repeated intravitreal injection of bevacizumab was performed if there was fluoroangiographic evidence of persistent or increased lesion activity (late-phase leakage) or increased lesion size compared with baseline angiograms (early-phase ICG angiograms).
If advanced lesion occurred during the follow-up, the treatment was discontinued. At baseline, patients received intravitreal bevacizumab. Patients were instilled with ofloxacin 0.3% drops every 5 min four times before the injection. Topical anaesthesia was achieved by applying oxybuprocaine hydrochloride eye drops and one drop of 5% povidone iodine solution was applied to the eyelids and in the fornix, followed after 5 min by draping and insertion of lid speculum. Intravitreal injection of 1.25 mg bevacizumab in 0.05 ml was carried out using a 30-gauge needle.
After 8±2 days, PDT was administered according to the standard protocol with ICG angiographic guidance. A spot size was chosen to cover the RAP lesion with a 200-μm border. When possible, the fovea was not included in the treatment spot.
For statistical analysis, we considered data obtained at baseline, 1, 3, 6, and 9 months. All data are expressed as mean±SD. Comparisons among the mean values were made through one-way ANOVA with least-significant difference (LSD) post hoc test. Correlations among variables were studied with Spearman's index of linear correlation. The minimum criterion for tests of significance was P<0.05. The statistical analysis was conducted using a commercial software (SPSS for Windows, ver.12.0; SPSS sciences, Chicago, IL, USA).
Results
In total, 21 eyes of 18 patients with newly diagnosed RAP were enrolled. The mean age was 77 (range 65–86) years. Twelve patients were female and six men. All patients were Caucasian.
On the basis of the Yannuzzi classification criteria,1 there were 5 eyes out of 21 (24%) with RAP stage I lesions, 12 eyes (57%) with RAP stage II, and 4 eyes (19%) in stage III.
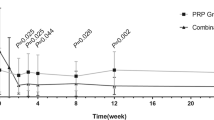
Mean logMAR BCVA showed no statistically significant differences during the follow-up examinations (P=0.10, ANOVA) (Table 1).
At 3 months from the injection, the BCVA improved by 3 or more lines in 3 of the 21 eyes (14%), remained stable in 72% of these eyes (15 of 21), and decreased 3 or more lines in 3 of the 21 eyes (14%). At 9 months, the BCVA improved by 3 or more lines in 14% of the eyes (3 of 21), remained stable in 12 eyes (57%), and worsened in 6 of 21 eyes (29%).
The mean values of the different OCT parameters evaluated for statistical analysis are reported in Table 2. Foveal thickness decreased significantly between baseline and all the follow-up visits (P<0.01, ANOVA). Max thickness decreased significantly between baseline and 1-month visits (P<0.01 LSD post hoc test), but increased significantly between 1- and 6-month visits (P<0.05 LSD post hoc test).
A significant difference between baseline and 1 month was shown by 3-mm thickness (P<0.05, ANOVA; P<0.01 LSD post hoc test). A 6-mm thickness and volume did not show any statistically significant difference among the scheduled visits (P=0.15 and P=0.33, respectively, ANOVA). All the considered OCT variables were significantly correlated among them (P<0.01, Spearman's test).
LogMAR BCVA showed a statistically significant correlation with foveal thickness and max thickness (P<0.01) and with 6- and 3-mm thickness (P<0.05).
At 1-month follow-up visit, 19 eyes out of 21 (90%) showed a complete resolution of leakage on fluorescein angiography and disappearance of the neovascular complex (early phase) or of the hot spot (late phase) on ICG angiography. One eye (5%) showed persistent leakage requiring re-treatment. One eye (5%) was not re-treated due to development of a disciform scar. At 3 and 6 months after treatment, 6 and 8 eyes out of 21 (29 and 38%, respectively) revealed the presence of leakage and re-appearance of neovascular net in ICG and were consequently re-treated (Figure 1). At the final scheduled visit, 33% (7 of 21) of eyes showed active lesions and patients were advised to receive an additional injection, 24% (5 of 21) of eyes presented a disciform scar.
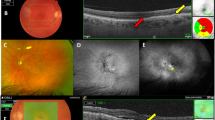
A 67-year-old patient with RAP (stage 2) in the right eye. Fluorescein angiogram (early phase), indocyanine green angiogram, fluorescein angiogram (late phase), horizontal and vertical OCT scan at baseline (a–e), 1 month (f–j), 6 months (k–o), and at 9 months (p–t). At baseline, a serous pigment epithelial detachment (PED) with retinal cystic changes could be observed. At 1 month after the combined treatment, PED and cystic changes resolved. At 6 months, because of the presence of leakage on the angiograms and of intraretinal fluid, a repeated IVT treatment was performed. At the last follow-up visit no leakage or intraretinal fluid was present.
A total of 36 intravitreal injections were given during the study with a mean of 1.7 injections per eye (range 1–3 injections per eye) (Table 3). Nine patients required two injections and three patients required three injections. No ocular or systemic adverse events were reported. A tear of the retinal pigment epithelium (RPE) was seen at the last follow-up visit in one studied eye re-treated at 6 months (Figure 2).
A 75-year-old patient with RAP (stage 2) in the left eye. Fluorescein angiogram (early phase), indocyanine green angiogram, fluorescein angiogram (late phase), horizontal and vertical OCT scan at baseline (a–e), 1 month (f–j), 6 months (k–o), and 9 months (p–t). Baseline examination revealed intraretinal fluid and leakage on angiograms, which resolved 1 month after the combined treatment. After 6 months, a recurrence was observed, with retinal haemorrhages, pigment epithelial detachment, and retinal cystic changes. A repeated IVT treatment was performed. At 9 months, a tear of the retinal pigment epithelium occurred.
Discussion
Despite numerous publications on the management of this distinct form of neovascular ARMD, to date no firm method of treatment for RAP has been established. Several uncontrolled case series have reported either an unfavourable outcome or conflicting data after photocoagulation, transpupillary thermotherapy, posterior sub-Tenon's space injection of anecortave acetate, and surgical approaches.5, 6, 7, 8, 9 Poor anatomical and functional results after PDT for RAP are also reported in the literature:10 the authors speculated that leakage of verteporfin into the retina would have the theoretical risk of inducing photooxidative damage (to the retina) when the molecule is activated and concluded that monotherapy was inadvisable. To resolve intraretinal oedema and therefore decrease the leakage of verteporfin into the cystoids spaces, intravitreal injection of triamcinolone followed by PDT has also been tried, showing promising results.11, 12 However, some investigators observed that such procedures may be associated with significant atrophy of the RPE and choriocapillaris in patients who developed recurrent exudation requiring re-treatments.13, 17, 21, 22 In addition, retinal toxicity of intravitreal triamcinolone cannot be entirely ruled out. An extensive damage of the photoreceptor outer segment and the RPE in pigmented rabbits after intravitreal triamcinolone acetonide is reported by Yu et al23, suggesting dose-related toxic effects of the drug.
Finally, the risk of developing increased intraocular pressure and the inevitable development of posterior subcapsular cataracts in phakic patients are the expected complications of triamcinolone acetonide.
Most recently, encouraging short-term results with intravitreal bevacizumab as monotherapy have been reported. Reductions of leakage, significant decrease in macular thickness and improvement or stabilization of visual acuity in about 90% of treated eyes were the common findings at the end of the follow-up. However, 30–50% of the treated eyes needed re-injection of bevacizumab within 3 months13, 14, 15 and 70% after the 3 monthly scheduled injections.16 Persistent lesion activity was noted in nearly all patients at the end of follow-up. In these studies, the high rate of required re-treatments might have enabled better visual outcomes compared with our treatment strategy. However, reported results should be interpreted with caution as most of these studies using bevacizumab as monotherapy did not include RAP stage, lesion size, and location.
A recent retrospective case series by Saito et al18 has documented that combined intravitreal bevacizumab and PDT effectively maintained or improved visual acuity in about 90% of treated eyes without requiring additional injections for 6 months. Lo Giudice et al19 in a pilot study have obtained analogous functional results with a mean number of injections for the 9 months of follow-up of 3.2±0.4.
In our protocol, we decided to perform intravitreal injection of bevacizumab before PDT to induce resolution of intraretinal oedema and therefore decrease the leakage of verteporfin into the cystoids spaces, minimizing the theoretical risk of inducing retinal photooxidative damage when the molecule is activated. Re-treatment consisted of intravitreal bevacizumab monotherapy to minimize the following hypothetical risks associated with multiple combo treatments:
-
Photothrombotic effect of the PDT on the physiological choroidal vessels enhanced by the VEGF blockage caused by bevacizumab.21
-
Possible high affinity of the verteporfin with the fibrin that is accompanying RAP, as postulated by Freund et al12, with the related risk of increasing its concentration in the irradiated area; this, in combination with VEGF blockage, may further increase the photochemical stress of the treatment resulting in choriocapillaris ischaemia.
-
Significant macular RPE and choriocapillaris atrophy following combined intravitreal triamcinolone acetonide and PDT for RAP, as recently reported.13, 22
Moreover, to minimize the ‘off-label’ use of an unproven agent, re-treatment criteria were based on angiographic findings rather then following a fixed dosing regimen as in ranibizumab studies or in other pilot studies.16, 24
Functionally, in this study, most of our patients had stabilization (72%) or improvement of three or more lines (14%) in vision after 3 months. This tendency was also observed after 9 months (57 and 14%, respectively). Anatomically, OCT data revealed a significant improvement at 1 month and subsequent regression towards baseline over the 9-month follow-up, although retinal thickness remained under the baseline values for the entire follow-up.
Also noteworthy of our study was the low rate of additional treatment with bevacizumab during the 9-month follow-up (1.7). During the first year of the PrONTO Study, the patients with RAP lesions received a mean 7.1 injections of ranibizumab as monotherapy in 12 months. Aware of several differences between the design of the two pivotal trials and between anti-VEGF drugs used that limit such comparisons, our results seem to suggest a possible benefit of the combo therapy also in the rate of intravitreal re-injections.25 However, in this study, the re-treatment rate (15 re-injections over the 9-month follow-up) significantly differs from the one recently reported by other authors.16, 17, 18, 19 Obviously, the difference in the study design (for example, sequence of combined treatments, duration of follow-up, number of patients, criteria for re-treatments, or different population included) may explain different rates of re-injections or functional results.26
In our study, re-treatment criteria based on angiographic findings could have affected the re-treatment rate. Using different criteria for monitoring and re-treatment, such as visual acuity outcomes and OCT, a better functional outcome would have probably been achieved at the expense of a higher re-injections rate.
With regard to safety, there was no evidence of macular atrophy, uveitis, endophthalmitis, ocular toxicity, or systemic thromboembolic events.
There are several obvious limitations to this pilot study. The sample size is small, the follow-up period is short, the series is uncontrolled, and the ideal interval between injection and PDT is not yet defined. Moreover, the most advantageous number and frequency of intravitreal bevacizumab injections to maintain the initial effect of the combo therapy remained uncertain. A loading dose with two subsequent monthly intravitreal bevacizumab injections might be performed to consolidate the functional and anatomical effect of the combined treatment as suggested by other authors.19 Finally, the dosage of intravitreal bevacizumab used has not been formally evaluated. We used a dosage of 1.25 mg bevacizumab in 0.05 ml, which is the most commonly reported in other studies and it is roughly equivalent to the same number of protein molecules of 0.5 mg ranibizumab.27
In conclusion, these data suggest that a possible synergistic effect may arise from the combination of intravitreal bevacizumab with verteporfin PDT for the treatment of RAP. Moreover, the use of combo therapy in a flexible strategy seems viable to obtain a functional and morphological stabilization in this type of neovascular lesion. However, BCVA and OCT may represent more suitable criteria for monthly monitoring of lesion activity. Our data also suggest a possible benefit of combo therapy in the rate of treatment of intravitreal injections. Nonetheless, prospective, randomized clinical trials to compare sequenced combined therapy with intravitreal bevacizumab and PDT to intravitreal bevacizumab as monotherapy for treatment of RAP are needed.
Summary

References
Viola F, Massacesi A, Orzalesi N, Ratiglia R, Staurenghi G . Retinal angiomatous proliferation: natural history and progression of visual loss. Retina 2009; 29: 732–739.
Yannuzzi LA, Negrao S, Iida T, Carvalho C, Rodriguez-Coleman H, Slakter J et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina 2001; 21: 416–434.
Gass JD, Agarwal A, Lavina AM, Tawansy KA . Focal inner retinal hemorrhages in patients with drusen. Retina 2003; 23: 741–751.
Freund KB, Ho IV, Barbazetto IA, Koizumi H, Laud K, Ferrara D et al. Type 3 neovascularization: the expanded spectrum of retinal angiomatous proliferation. Retina 2008; 28: 201–211.
Johnson TM, Glaser BM . Focal laser ablation of retinal angiomatous proliferation. Retina 2006; 26: 765–772.
Kuroiwa S, Arai J, Gaun S, Iida T, Yoshimura N . Rapidly progressive scar formation after transpupillary thermotherapy in retinal angiomatous proliferation. Retina 2003; 23: 417–420.
Klais CM, Eandi CM, Ober MD, Sorenson JA, Sadeghi SN, Freund KB et al. Anecortave acetate treatment for retinal angiomatous proliferation: a pilot study. Retina 2006; 26: 773–779.
Nakata M, Yuzawa M, Kawamura A, Shimada H . Combining surgical ablation of retinal inflow and outflow vessels with photodynamic therapy for retinal angiomatous proliferation. Am J Ophthalmol 2006; 141: 968–970.
Sakimoto S, Gomi F, Sakaguchi H, Tano Y . Recurrent retinal angiomatous proliferation after surgical ablation. Am J Ophthalmol 2005; 139: 917–918.
Boscia F, Furino C, Sborgia L, Reibaldi M, Sborgia C . Photodynamic therapy for retinal angiomatous proliferations and pigment epithelium detachment. Am J Ophthalmol 2004; 138: 1077–1079.
Nicolo M, Ghiglione D, Lai S, Calabria G . Retinal angiomatous proliferation treated by intravitreal triamcinolone and photodynamic therapy with verteporfin. Graefes Arch Clin Exp Ophthalmol 2006; 244: 1336–1338.
Freund KB, Klais CM, Eandi CM, Ober MD, Goldberg DE, Sorenson JA et al. Sequenced combined intravitreal triamcinolone and indocyanine green angiography-guided photodynamic therapy for retinal angiomatous proliferation. Arch Ophthalmol 2006; 124: 487–492.
Meyerle CB, Freund KB, Iturralde D, Spaide RF, Sorenson JA, Slakter JS et al. Intravitreal bevacizumab (Avastin) for retinal angiomatous proliferation. Retina 2007; 27: 451–457.
Ghazi NG, Knape RM, Kirk TQ, Tiedeman JS, Conway BP . Intravitreal bevacizumab (Avastin) treatment of retinal angiomatous proliferation. Retina 2008; 28: 689–695.
Joeres S, Heussen FM, Treziak T, Bopp S, Joussen AM . Bevacizumab (Avastin) treatment in patients with retinal angiomatous proliferation. Graefes Arch Clin Exp Ophthalmol 2007; 245: 1597–1602.
Gharbiya M, Allievi F, Recupero V, Martini D, Mazzeo L, Gabrieli CB . Intravitreal bevacizumab as primary treatment for retinal angiomatous proliferation: twelve-month results. Retina 2009; 29: 740–749.
Ladewig MS, Karl SE, Hamelmann V, Helb HM, Scholl HP, Holz FG et al. Combined intravitreal bevacizumab and photodynamic therapy for neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2008; 246: 17–25.
Saito M, Shiragami C, Shiraga F, Nagayama D, Iida T . Combined intravitreal bevacizumab and photodynamic therapy for retinal angiomatous proliferation. Am J Ophathalmol 2008; 146: 935–941.
Lo Giudice G, Gismondi M, De Belvis V, Cian R, Tavolato M, Galan A . Single-session photodynamic therapy combined with intravitreal bevacizumab for retinal angiomatous proliferation. Retina 2009; 29: 949–955.
Vujosevic S, Midena E, Pilotto E, Radin PP, Chiesa L, Cavarzeran F . Diabetic macular edema: correlation between microperimetry and optical coherence tomography findings. Invest Ophthalmol Vis Sci 2006; 47: 3044–3051.
Rouvas AA, Papakostas TD, Ladas ID, Vergados I . Enlargement of the hypofluorescent post photodynamic therapy treatment spot after a combination of photodynamic therapy with an intravitreal injection of bevacizumab for retinal angiomatous proliferation. Graefes Arch Clin Exp Ophthalmol 2008; 246: 315–318.
Sutter FK, Kurz-Levin MM, Fleischhauer J, Bösch MM, Barthelmes D, Helbig H . Macular atrophy after combined intravitreal triamcinolone acetonide (IVTA) and photodynamic therapy (PDT) for retinal angiomatous proliferation (RAP). Klin Monatsbl Augenheilkd 2006; 223: 376–378.
Yu SY, Damico FM, Viola F, D’Amico DJ, Young LH . Retinal toxicity of intravitreal triamcinolone acetonide: a morphological study. Retina 2006; 26: 531–536.
Antoszyk AN, Tuomi L, Chung CY, Singh A, FOCUS Study Group. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results. Am J Ophthalmol 2008; 145: 862–874.
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007; 143: 566–583.
Gupta BM, Jyothi S, Sivaprasad S . Current treatment options for retinal angiomatous proliferans (RAP). Br J Ophthalmol published online November 5, 2009. [Epub ahead of print]
Rich RM, Rosenfeld PJ . Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina 2006; 26: 495–511.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Viola, F., Mapelli, C., Villani, E. et al. Sequential combined treatment with intravitreal bevacizumab and photodynamic therapy for retinal angiomatous proliferation. Eye 24, 1344–1351 (2010). https://doi.org/10.1038/eye.2010.33
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.33
Keywords
This article is cited by
-
Therapie der retinalen angiomatösen Proliferation im Stadium III
Der Ophthalmologe (2013)
-
One-year results of bevacizumab intravitreal and posterior sub-Tenon injection of triamcinolone acetonide with reduced laser fluence photodynamic therapy for retinal angiomatous proliferation
Japanese Journal of Ophthalmology (2012)