Abstract
Aims and purpose
To identify patterns and rates of adherence with travoprost eye drops using the Travatan dosing aid (TDA) and to present a method for graphically presenting adherence data.
Methods
A prospective observational cohort study of patients on travoprost (prostaglandin) monotherapy. Patients were dispensed a TDA and followed up after approximately 3 months of usage. Data were downloaded from the TDA into a computer for analysis. Analysis used inter-dose intervals (the time between each dosing) to look at adherence between days 4 and 75.
Results
In all, 100 patients were invited to participate, 53 agreed and complete TDA data sets were obtained from 37. In total 23 of the complete data sets showed good adherence (dosing within ±4 h of the agreed dosing time on >80% of occasions), 3 patients discontinued usage before 75 days, 4 showed frequent drug holidays (no dosing for ⩾8 days) and 7 frequently missed doses with adherence rates of <60%. Of the 16 patients for whom no TDA data was obtained, 5 were lost to follow-up, 4 had faulty/damaged TDAs, 3 changed medication, 3 preferred not to use the TDA, and 1 was hospitalized.
Conclusions
There were four easily defined patterns of adherence; (1) good adherence; (2) discontinued usage; (3) frequent drug holidays; and (4) frequent missed doses with low adherence rates. A new method for graphically presenting adherence data helps clinicians identify the pattern of usage and is a valuable aid to the overall management of patients on travoprost therapy.
Similar content being viewed by others
Introduction
Despite advances in the development of new therapies and a better understanding of the pathology of glaucoma, a significant number of patients continue to show disease progression. An important factor in controlling the rate of progression is the patient's adherence (the extent to which patient continues an agreed-on mode of treatment1) and persistence (the duration from commencement to discontinuation of treatment1) with medical therapy; however, accurate and reliable measures of adherence are rarely available during management review and the ability of clinicians to identify poor adherence is poor.2, 3 Management decisions are thus often based upon presenting IOP levels and self reported compliance/adherence. In such instances the decision to alter medical therapy or proceed with surgical intervention may be based on a poor understanding of the habitual IOP and the efficacy of current therapy.
In a systematic review by Olthoff4 non-adherence to medical therapy for glaucoma ranged from 4.6 to 80% across 34 studies. Most of these studies used patient self-reports of adherence or pharmacy claims data. Both these modalities have important shortcomings and have been shown to be less accurate than electronic monitoring.5
Although devices for the electronic monitoring of adherence have been reported upon for some time, very few devices have been made widely available to the clinical ophthalmologist. However, recently Alcon has introduced the Travatan dosing aid (TDA; Alcon Laboratories, Fort Worth, TX, USA), which electronically stores data on the time, date, and number of drops administered, which can be downloaded into a computer at a later date (see Cronin6 for a full description and Figure 1). Early reports have shown that this device accurately records drop administration and that adherence rates are in the order of 75%.6, 7, 8, 9 All previous publications on electronic monitoring devices have used global indices to describe adherence over a predetermined time period, for example adherence rate, percent of doses. One of the major advantages of electronic monitoring is the provision of information on the time and date of each dose. This information allows the clinician to differentiate between different patterns of loss, for example between a patient who discontinued usage after a period of time from one who is forgetful and regularly misses doses. Global indices do not allow such differentiation.
The purpose of this prospective observational study was to identify patterns of adherence with the TDA in a representative population of patients attending a major NHS eye hospital and to present a new method for viewing adherence data that retains clinically important information on the timing of doses.
Materials and methods
Patient selection
This is a prospective observation cohort study of consecutive patients attending Manchester Royal Eye Hospital (MREH) outpatient clinics with primary open angle glaucoma, ocular hypertension, or chronic narrow angle glaucoma.
An inclusion criterion was that the patient had been on established travoprost monotherapy for ⩾3 months before the onset of the study. Patients were excluded if they were unable to provide informed consent, had comorbidity including acute angle closure glaucoma or were on multiple medications for glaucoma.
Investigations
At an initial interview patients answered a simple questionnaire, were instructed on how to use the TDA (see Figure 1), and had their IOP recorded along with a disc assessment.
The questionnaire was administered by the researcher and included questions on the patient's time of drop instillation, an estimate of the average number of drops they missed per month and the reasons for the same.
Baseline demographic data including, age, gender, ethnicity, baseline untreated IOP of each eye, and visual field parameters (mean deviation) were obtained from patient records.
The TDA records the date and time of drop instillation every time the lever on the TDA is depressed. The subjects were made aware of this and they knew that they were being monitored. Patients were advised to continue with their regular drug administration as instructed by the treating clinician. The TDA also includes visual and auditory reminders of when a drop is due. The visual reminder flashes a symbol on the side of the drop aid when a dose is due and the audible reminder mechanism issues an intermittent tone when a drop is due. Each TDA is programmed before dispensing with the timing of dosing and whether or not to activate the audible reminder. The audible reminder was turned off for all patients enrolled in this study. The visual reminder was left on there being no way to turn it off with the provided software. Patients were instructed to contact the research team should they have any problems with the TDA.
The patients were reviewed after approximately 3 months when the TDA data was downloaded into a computer for analysis. No patients were removed from the study, once recruited, irrespective of any problems encountered. The initial questionnaire was administered again with additional questions on the acceptability of the TDA.
Patients who failed to attend the review appointment were contacted by phone and, whenever possible, repeat appointments booked. In instances where mobility or transport problems were cited as cause for non-attendance, a researcher visited their homes to collect TDA data and administer the questionnaire. The cases where the researcher was not able to get the data were classified as incomplete.
Malfunctioning units, as reported by the patients, were collected and an attempt was made to extract any saved data and restart the patient with a new TDA.
Analysis was based on the data collected from days 4 (after the patient had time to get used to the TDA) to 75 (shortest follow-up time). No specific benchmark was set as to what constitutes adequate adherence but instead a plot of the inter-dose interval was produced for each patient. Repeat/multiple measures within a 4-h time window were treated as a single dose to overcome the problems of multiple extra recordings highlighted by Friedman8 with the TDA. The study was approved by the Tameside and Glossop Research Ethics Committee and informed consent was obtained from all recruited subjects. All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Results
Study recruitment started in January 2008 and ended in December 2008. A total of 100 patients were invited to participate, of which 53 were subsequently recruited (see Figure 2). The average age of the recruited patients was 67 years (minimum 39, maximum 95) and there were 25 (47%) women and 28 (53%) men. 46 were Caucasians, 5 of African descent, 1 Indian, and 1 Asian.
Drop aid data were collected from 37 patients. Of the 16 patients in whom TDA data were not obtained, we were unable, even after repeated attempts, to get 5 patients to attend the hospital and return the drop aid. On four occasions the drop aid failed. In one case the aid was dropped in the bath and in another a child got hold of it and removed the battery. On three occasions the patient's medication was changed and as the aid is specific to the Alcon range of medications the patient was removed from the trial. A total of 3 patients found it easier not to use the drop aid, one reporting that it was more cumbersome than the bottle on its own. One patient was admitted to hospital with a serious illness and discounted using the aid.
There were various patterns of usage detected with the drop aid. Figure 3 gives four examples of how each patient's inter-dose interval changed during the study period. A perfectly adherent patient would have a constant inter-dose interval of 1 day. Values above 1 represent delayed or missed doses and values below 1 represent a shortened inter-dose interval. Also included in this figure is the number of cases that fell within each of the four patterns of use.
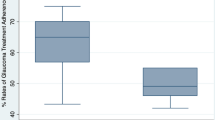
Median adherence rates for the 34 patients who continued to use the TDA for 75 days are given for various time windows (period either side of median dosing time when administration of drop was counted as adherent) in Figure 4. For this group of patients the adherence increased with the time window, stabilizing at about ±4 h with a median value of 80–85%. Within this group of 34 patients there were 3 whose adherence was poor, <40%. These 3 patients fell into type three of Figure 3 (frequently missed doses often for several consecutive days). Only one of these patients showed an increasing trend for missed doses with time. Within the whole group there was a small fall in adherence with time. On average the 34 patients were 96% adherent during the first 10 days and this fell to 86% after 30 days and remained close to this value for the rest of the study period.
Outcomes for all patients entered in to the trial including adherence rates for 34 patients who used the drop aid for 75 days. Median adherence rates and the distribution of adherence rates are given in the box plot for various time windows (period either side of median dosing time when administration of drop was counted as adherent).
Patients were asked about their adherence at the exit interview. They were asked how many drops they missed per month (0, 1–3, 4–7, or 8–10). Figure 5 shows how the patient's estimate of their adherence compares with that of the drop aid. The majority of patients overestimate their rate of adherence, some quite markedly.
There was no obvious relationship between adherence and age, with the duration of medication or with gender.
Discussion
This study has shown that of those patients who completed 75 days of TDA usage the median adherence rate was greater than 80%, when adherence is defined as within ±4 h of the median dosing time. This finding is similar to that of other groups reporting on the TDA.10, 11 However, this figure of >80% adherence does not adequately describe adherence within our whole cohort. Of the 34 patients who completed 75 days with the TDA, 3 had adherence rates of less than 40% and there were 3 further patients whose TDA data indicated that they stopped all medication after 3, 37, and 49 days, respectively. In addition there were 5 patients who were lost to follow-up, in whom it is likely that the adherence was poor. If we exclude the additional 11 patients who discontinued the TDA for a variety of reasons unlikely to be related to adherence (malfunction, drug switches, hospitalization, and so on) our results suggest that 26% (11 out of 42) had very poor adherence. The TDA has been valuable in not only identifying those patients who are at increased risk of progressive loss, which can lead to a better targeting of resources, but also in identifying different patterns of adherence that can be used in future management. For example, in those cases that discontinue after a short time, offer early surgery or laser. Those that have frequent holidays, explore with them whether this results from difficulties in getting repeat prescriptions, or whether this results from shift work patterns and in those that have frequent lapses, whether additional prompts (eg, the audio-facility) would aid their adherence or whether their lapses are so frequent and long that they should be offered early surgery as in the first group.
We found a small drop in adherence within the evaluation period that seemed to level off after about 40 days. Longer trials are necessary to establish whether adherence is maintained in the longer term and the impact of various factors upon adherence, for example different review intervals. The TDA can provide valuable data for such studies and for studies of other factors such as the benefits of patient education/knowledge.
We found that patient reported adherence was below that of the TDA in the majority of cases. In some instances patients reported missing no drops when their TDA indicated <40% adherence. Similar disparities between reported and objective measures of adherence have been given by Okeke10 and Kass2 and this paper confirms their conclusions, that relying upon patient reports of adherence is prone to error.
Study limitations include data collection from a single inner city eye hospital, which is unlikely to be representative of the UK NHS population as a whole. Also, the sample size, although being sufficient to provide examples of different adherence patterns, was insufficient to provide accurate measures of proportions or to look at the relationships between patients’ characteristics and adherence. Patients were aware that their adherence was being monitored during the study and this in itself might have influenced the results, the Hawthorne effect.12
We chose to present individual adherence data graphically as inter-dose interval vs time. Mean inter-dose intervals have previously been used by Robin13 in a study of adherence with different drop regimes, but to our knowledge this is the first time that a graphical representation of the inter-dose interval has been used to classify adherence into different patterns. This format made it easy to pick out different patterns of adherence, for example, treatment holidays, declining adherence with time, and so on. The formats currently available with the dosing aid, log of dosing times, and calendar report, even though helpful, were not found to be as easy to interpret. Our presentation method could easily be incorporated into the software of the drop aid to help the clinician with management decisions.
The TDA can provide valuable data on the pattern and rate of adherence to medical therapy with Travaprost. Four different patterns of adherence were found when plotting the inter-dose interval: discontinued dosing; good adherence; dosing holidays; and frequent lapses. The TDA has the potential to act as a valuable management aid for glaucoma and ocular hypertension.

References
Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA et al. Medication compliance and persistence: terminology and definitions. Value Health 2008; 11: 44–47.
Kass MA, Meltzer DW, Gordon M, Cooper D, Goldberg J . Compliance with topical pilocarpine treatment. Am J Ophthalmol 1986; 101: 515–523.
Kass MA, Gordon M, Meltzer DW . Can ophthalmologists correctly identify patients defaulting from pilocarpine therapy? Am J Ophthalmol 1986; 101: 524–530.
Olthoff CM, Schouten JS, van de Borne BW, Webers CA . Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension: an evidence-based review. Ophthalmology 2005; 112: 953–961.
Farmer KC . Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther 1999; 21: 1074–1090.
Cronin TH, Kahook MY, Lathrop KL, Noecker RJ . Accuracy and performance of a commercially available dosing aid. Br J Ophthalmol 2007; 91: 497–499.
Boden C, Sit A, Weinreb RN . Accuracy of an electronic monitoring and reminder device for use with travoprost eye drops. J Glaucoma 2006; 15: 30–34.
Friedman DS, Jampel HD, Congdon NG, Miller R, Quigley HA . The TRAVATAN dosing aid accurately records when drops are taken. Am J Ophthalmol 2007; 143: 699–701.
Friedman DS, Okeke CO, Jampel HD, Ying GS, Plyler RJ, Jiang Y et al. Factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology 2009; 116: 1097–1105.
Okeke CO, Quigley HA, Jampel HD, Ying GS, Plyler RJ, Jiang Y et al. Adherence with topical glaucoma medication monitored electronically. The Travatan Dosing Aid Study. Ophthalmology 2009; 116: 191–199.
Stein JD, Ayyagari P, Sloan FA, Lee PP . Rates of glaucoma medication utilization among persons with primary open-angle glaucoma, 1992–2002. Ophthalmology 2008; 115: 1315–1319.
De Amici D, Klersy C, Ramajoli F, Brustia L, Politi P . Impact of the Hawthorne effect in a longitudinal clinical study: the case of anesthesia. Control Clin Trials 2000; 21: 103–114.
Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS . Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol 2007; 144: 533–540.
Acknowledgements
We thank Alcon for generously providing the Travatan Dosing Aids. This study was supported by the Manchester Academic Health Sciences Centre (MAHSC) and the NIHR Manchester Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ajit, R., Fenerty, C. & Henson, D. Patterns and rate of adherence to glaucoma therapy using an electronic dosing aid. Eye 24, 1338–1343 (2010). https://doi.org/10.1038/eye.2010.27
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.27
Keywords
This article is cited by
-
Factors affecting medication adherence among older adults using tele-pharmacy services: a scoping review
Archives of Public Health (2022)
-
Personalized behavior change program for glaucoma patients with poor adherence: a pilot interventional cohort study with a pre-post design
Pilot and Feasibility Studies (2018)
-
Improving adherence to glaucoma medication: a randomised controlled trial of a patient-centred intervention (The Norwich Adherence Glaucoma Study)
BMC Ophthalmology (2014)
-
Patterns of adherence behaviour for patients with glaucoma
Eye (2013)
-
Lazy Sunday afternoons: the negative impact of interruptions in patients’ daily routine on adherence to oral antidiabetic medication. A multilevel analysis of electronic monitoring data
European Journal of Clinical Pharmacology (2013)