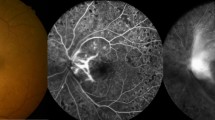
Abstract
Purpose
To evaluate the effect of intra-silicone injection of bevacizumab for the treatment of neovascular glaucoma (NVG) after vitrectomy for advanced proliferative diabetic retinopathy.
Methods
Bevacizumab was injected into the silicone oil in five pseudophakic eyes of five patients with NVG. The iris neovascularization (INV) and NVG had developed 1.5–4 months after vitrectomy and silicone oil tamponade. The main outcome measures were regression of INV, intraocular pressure and visual acuity.
Results
In all eyes, INV regressed and intraocular pressure was controlled within 7 days. Visual acuity improved in all eyes. In one patient, INV and NVG recurred 10 weeks after the injection and was successfully treated with a repeat intra-silicone bevacizumab injection.
Conclusion
Intra-silicone injection of bevacizumab is effective in the treatment of patients with INV and NVG after vitrectomy for advanced proliferative diabetic retinopathy.
Similar content being viewed by others
Introduction
Vascular endothelial growth factor (VEGF) plays a central role in several ocular pathologies characterized by neovascularization and increased vascular permeability.1 Neovascular glaucoma (NVG) is a devastating complication associated with ischaemic retinopathies such as diabetic retinopathy.2 Standard treatment includes retinal photocoagulation and cyclodestructive or drainage procedures.3 Several studies have reported the value of intraocular anti-VEGF therapy with bevacizumab as a treatment for iris neovascularization (INV) associated with glaucoma.4, 5, 6, 7
Silicone oil is an important adjunct in the management of complex vitreoretinal surgical procedures. It has been extensively used as a tamponade in cases in which conventional vitreoretinal surgery is likely to result in a poor success rate.8 In the presence of silicone oil tamponade, the delivery and concentration of drugs injected into the posterior segment of the eye become unpredictable.
The aim of this pilot study was to investigate the value of intraocular anti-VEGF therapy with bevacizumab as a treatment for INV associated with glaucoma after vitrectomy and silicone oil tamponade for the treatment of advanced diabetic retinopathy.
Patients and methods
During a 12-month period, five eyes of five consecutive patients with the entry criterion of clinical INV associated with NVG were prospectively studied. They had earlier undergone vitreoretinal surgery, including complete panretinal endophotocoagulation and silicone oil endotamponade for the treatment of severe proliferative diabetic retinopathy (PDR). All patients received injections of 2.5 mg bevacizumab (0.1 mg of commercially available Avastin solution; Genentech Inc. (made for F Hoffmann-La Roche Ltd, Basel, Switzerland)) into the silicone-filled vitreous cavity. Patients had to have at least 3 months of follow-up after intra-silicone injection of bevacizumab for inclusion in the study.
Exclusion criteria were the presence of any tractional and/or rhegmatogenous retinal detachment, active intraocular inflammation or signs of earlier inflammation, such as iris-intraocular lens synechiae or clinically obvious macular oedema, and intraocular pressure (IOP) of more than 22 mm Hg before the appearance of INV. Also, patients with more than 3 days of delay between INV detection and intraocular bevacizumab injection were excluded.
The characteristics of the disease, lack of any treatment other than cycloablation, the experimental nature of the study, and the off-label use of bevacizumab were explained to the patients and informed consent was obtained. The institutional review board/ethics committee of the Eye Research Center approved the study.
Ophthalmic examinations included visual acuity testing, slit lamp biomicroscopy, measurement of IOP, and dilated funduscopy. Paraclinical evaluation included slit lamp and fundus photography. The patients were visited on the first day after injection and at 3 days, 1 week, and monthly thereafter depending on the involution of INV and IOP control. The examination protocol was repeated for all post-operative visits.
Results
Five pseudophakic eyes with a mean age of 62.4±7.6 years were studied. Table 1 shows the patient characteristics. The INV and NVG developed 1.5–4 months after vitrectomy and silicone oil tamponade. In all cases, the INV completely disappeared clinically within 7 days, starting almost 72 h after injection. Visual acuity improved in all eyes. The mean visual acuity improved from 1.7±0.2 LogMAR before injections to 1.5±0.3 LogMAR at the final examination (P=0.02). The mean IOP before intra-silicone injections was 34 mm Hg (range 25–45) despite maximum anti-glaucoma therapy. In all cases, the IOP decreased within 72 h and returned to levels <20 mm Hg with timolol and dorzolamide within 7 days. No inflammation or other complications were observed. INV recurrence associated with IOP elevation was detected in one patient 10 weeks after injection, which regressed after intra-silicone reinjection of bevacizumab.
Discussion
Intraocular administration of bevacizumab has been shown to induce rapid regression of neovascularization in diabetic eyes.4, 7 It may be an advantageous treatment option in the eyes in which fundus-obscuring cataract or vitreous haemorrhage prevents photocoagulation, and in eyes in which INV is not halted by panretinal photocoagulation.3, 4, 7
INV and NVG are rarely encountered after vitreoretinal surgery for complications of PDR. So far, no treatment has been available for regression of neovascularization in these patients because retinal photocoagulation has already been carried out before and/or during the surgery. Therefore, the treatment of NVG in these patients has been mainly limited to cycloablative procedures as drainage procedures are often unsuccessful in the presence of active NVI.3
We observed a uniformly good response to intra-silicone injection of bevacizumab in this series of five patients with NVG after vitreoretinal surgery for advanced PDR. We therefore conclude that this injection may be the treatment of choice for these patients, and we recommend it as soon as possible after the appearance of active INV and NVG, as delayed treatment may result in peripheral anterior synechiae formation complicating the clinical picture.
The rapid diffusion of bevacizumab out of silicone oil, as evidenced by dramatic clinical response in our patients, also has other important implications. Many vitreoretinal surgeons inject bevacizumab intravitreally at the end of vitrectomy for PDR without tamponade.9 Our results indicate that it can also be injected at the end of surgery for severe cases where silicone tamponade is used, and it may help to induce regression of the remaining new vessels as well as serve as an anti-inflammatory agent.10
We injected 2.5 mg bevacizumab into the silicone oil instead of the more commonly used dose of 1.25 mg. The 2.5-mg dose has been used by several investigators for the treatment of choroidal neovascularization and diabetic macular oedema, and has been found to have the same or higher efficacy as compared with the 1.25-mg dose, without significant side effects.11, 12, 13 In addition, we were not certain about the efficacy of bevacizumab injected inside the silicone oil, and therefore we thought we might have more bevacizumab molecules reaching the retinal periphery with this higher dose. It is quite likely that the regular 1.25 mg dose may have the same efficacy in regressing INV.
Another point to be considered is that bevacizumab, which diffuses out of the silicone oil, may accumulate in the inferior meniscus of the fluid underneath silicone oil by gravity. We cannot speculate about the concentration of bevacizumab in this fluid meniscus, but we did not observe any sign of clinical toxicity in these patients. Concentrations up to 5 mg in the vitreous cavity have not been associated with histologic or electroretinographic signs of toxicity in rabbit eyes.14 Our group has studied the effects of higher doses of intravitreal bevacizumab injections in rabbit eyes and did not observe any sign of toxicity after injections up to 7.5 mg (Modarres et al., unpublished data).
The small size of this study is partly because of the fact that the occurrence of NVG in post-diabetic vitrectomy eyes with silicone oil is a rarely observed complication. Larger studies with longer follow-up are required to further validate these findings.
References
Ferrara N . Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004; 25: 581–611.
Beer PM, Wong SJ, Hammad AM, Falk NS, O’Malley MR, Khan S . Vitreous level of unbound bevacizumab and unbound vascular endothelial growth factor in two patients. Retina 2006; 26: 871–876.
Sivak-callcott JA, O'Day DM, Gass DM, Tsai JC . Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology 2001; 108: 1767–1776.
Oshima Y, Sakaguchi H, Gomi F, Tano Y . Regression of iris neovascularization after intravitreal injection of bevacizumab in patients with proliferative diabetic retinopathy. Am J Ophthalmol 2006; 142: 155–158.
Cheng JYC, Wong DWK, Ang CL . Intraocular Avastin (bevacizumab) for neovascularisation of the iris and neovascular glaucoma. Ann Acad Med Singapore 2008; 37: 72–74.
Grisanti S, Biester S, Peters S, Tatar O, Ziemssen F, Bartz-Schmidt KU, The Tuebingen Bevacizumab Study Group. Intracameral bevacizumab for iris rubeosis. Am J Ophthalmol 2006; 142: 158–160.
Wakabayashi T, Oshima Y, Sakaguchi H, Ikuno Y, Miki A, Gomi F et al. Intravitreal bevacizumab to treat iris neovascularization and neovascular glaucoma secondary to ischemic retinal diseases in 41 consecutive cases. Ophthalmology 2008; 115 (9): 1571–1580.
Gallemore RP, McCuen BW . Silicone oil in vitreoretinal surgery. In: Ryan SJ (ed.) Retina, 4th edition. St Louis: Mosby, 2006; pp 2211–2234.
Avery RL . Intravitreal bevacizumab in the surgical treatment of proliferative diabetic retinopathy. Retina today, March/April 2008; 50–53.
Kiss C, Michels S, Prager F, Weigert G, Geitzenauer W, Schmidt-Erfurth U . Evaluation of anterior chamber inflammatory activity in eyes treated with intravitreal bevacizumab. Retina 2006; 26 (8): 877–881.
Costa RA, Jorge R, Calucci D, Cardillo JA, Melo Jr LA, Scott IU . Intravitreal bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): results of a phase 1 dose-escalation study. Invest Ophthalmol Vis Sci 2006; 47: 4569–4578.
Wu L, Arevalo JF, Roca JA, Maia M, Berrocal MH, Rodriguez FJ et al. Comparison of two doses of intravitreal bevacizumab (Avastin) for treatment of macular edema secondary to branch retinal vein occlusion: results from the Pan-American Collaborative Retina Study Group at 6 months of follow-up. Retina 2008; 28: 212–219.
Modarres M, Naseripour M, Falavarjani KG, Nikeghbali A, Hashemi M, Parvaresh MM . Intravitreal injection of 2.5 mg vs 1.25 mg bevacizumab (Avastin) for treatment of CNV associated with AMD. Retina 2009; 29 (3): 319–324.
Manzano RP, Peyman GA, Khan P, Kivilcim M . Testing intravitreal toxicity of bevacizumab (Avastin). Retina 2006; 26: 257–261.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Conflict of interest: None
Additional information
This work was presented in part at the AAO 2008 annual meeting
Rights and permissions
About this article
Cite this article
Falavarjani, K., Modarres, M. & Nazari, H. Therapeutic effect of bevacizumab injected into the silicone oil in eyes with neovascular glaucoma after vitrectomy for advanced diabetic retinopathy. Eye 24, 717–719 (2010). https://doi.org/10.1038/eye.2009.94
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.94
Keywords
This article is cited by
-
Analysis of urgent follow up visits and complications after intravitreal injections: a retrospective cohort study
International Journal of Retina and Vitreous (2022)
-
Safety and complications of intravitreal injections performed in an Asian population in Singapore
International Ophthalmology (2017)
-
Anti-VEGF therapy in symptomatic peripheral exudative hemorrhagic chorioretinopathy (PEHCR) involving the macula
Graefe's Archive for Clinical and Experimental Ophthalmology (2016)
-
Intrasilicone oil injection of bevacizumab at the end of retinal reattachment surgery for severe proliferative vitreoretinopathy
Eye (2014)
-
Pharmakokinetik intravitreal applizierter VEGF-Inhibitoren
Der Ophthalmologe (2014)