Abstract
Purpose
To evaluate peripapillary retinal nerve fibre layer (RNFL) thickness and macular inner and outer retinal thickness using spectral domain optical coherence tomography (Sd-OCT) in patients with chronic exposure to hydroxychloroquine or chloroquine.
Methods
Subjects were divided into three groups: Group I, four patients with hydroxychloroquine or chloroquine toxicity with abnormal fundus; Group II, eight patients with chronic exposure without fundus changes; and Group III, eight visually normal controls. Peripapillary RNFL thinning for an individual quadrant was based on measurements of less than the 5th percentile from at least two out of four segments in the quadrant. Macular scans on Groups I and II were carried out to compare the thickness of the inner, outer, and full-thickness retina to that of Group III.
Results
The mean ages in Groups I, II, and III were 57.6±8.0, 54.9±11.0 and 53.7±10.5 years, respectively (P=0.83). Median (range) duration of exposure was 7.5 (5–12) years in Group I, and was 10 (6–35) years in Group II. Seven (88%) of eight eyes in Group I showed peripapillary RNFL thinning in at least one quadrant, whereas none of Groups II and III did so. Using macular scans, Group I showed significant thinning of the inner, outer, and full-thickness retina compared to Group III (P<0.001). Group II had significant thinning only of the inner retina compared to Group III (P<0.001).
Conclusions
OCT is useful to detect peripapillary RNFL thinning in clinically evident retinopathy, and selective thinning of the macular inner retina can be detected in the absence of clinically apparent fundus changes.
Similar content being viewed by others
Introduction
Chloroquine was first used as an antimalarial agent. It subsequently played an important therapeutic role in various rheumatologic diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and other inflammatory and dermatologic conditions. Retinal toxicity from chloroquine has been recognized for decades.1 The use of its analogue, hydroxychloroquine, has largely replaced chloroquine in most parts of the world, particularly in the United States, because of better tolerability at high dosages. Both medications share a similarity in their therapeutic and toxicologic aspects. Although retinal toxicity from hydroxychloroquine is infrequently seen,2 its use is still of concern due to potentially irreversible visual loss.3
The clinical sign of chloroquine or hydroxychloroquine toxicity is typically characterized by bilateral pigmentary change of the macula, often sparing the foveal centre, known as a bull's eye maculopathy. Retinal toxicity may be infrequently observed selectively in the more peripheral retina without significant macular changes.4, 5 Patients may complain of central visual loss, visual field defects, colour vision deficiency, photoaversion, night blindness, and entopic phenomena.1 Early detection of toxicity before fundus changes is vitally important because prompt discontinuation of these medications may reverse retinal toxicity at an early stage.
The precise mechanism of retinal toxicity remains unclear. Clinical characteristic changes imply that the distal retina (retinal pigment epithelium (RPE) and photoreceptors) might be primarily involved. Studies in mice also suggest that photoreceptor cells may be mainly affected with reversible inner retinal lipidosis and irreversible photoreceptor cell degeneration, which continues to progress despite cessation of medication.6, 7 An earlier report by Rosenthal et al8 indicated that chloroquine given parenterally in rhesus monkeys could cause significant inner and outer retinal changes shown in histopathological studies in the absence of clinical retinal findings on fundus photography, fluorescein angiography, or electrophysiologic testing. The initial pathological changes were observed in the retinal ganglion cell layer (GCL) with the accumulation of membranous cytoplasmic bodies followed by cellular degeneration. At a later stage, degenerative changes were also seen in the photoreceptors and the RPE cells, respectively. These changes in multilamellar structures were proposed to occur secondarily to an alteration of protein synthesis9, 10 and lipid peroxidation.11 Another study by Hallberg et al12 showed that effects on phospholipid metabolism after long-term chloroquine exposure in mice were evident selectively in the retinal ganglion cells, but neither in the RPE cells nor in the photoreceptor cells. These findings suggest that the retinal ganglion cells may be affected initially or primarily in the early course of retinal toxicity, and we speculate that the structure of the ganglion cells and their axons, the retinal nerve fibre layer (RNFL), may be compromised in patients taking these medications for a substantial period of time.
Hence, we used spectral domain optical coherence tomography (Sd-OCT) to measure the peripapillary RNFL thickness in patients with long-term exposure to hydroxychloroquine or chloroquine. Quantitative thickness measurements of the inner, outer, and full-thickness retina in the posterior pole using an automated algorithm were also carried out in the study subjects and results were compared with age-, gender- and race-similar healthy and visually normal control subjects.
Patients and methods
This study included patients with a history of the chronic use of chloroquine or hydroxychloroquine for at least 5 years. Study patients were divided into two groups: Group I with fundoscopic changes indicating toxicity and Group II without abnormal fundoscopic changes. All patients were examined by two authors (SP and GAF) in the Electrophysiology and Inherited Retinal Disease Unit at the Illinois Eye and Ear Infirmary. The patients were prospectively recruited from either this clinic or a comprehensive eye clinic at the University of Illinois. Seven patients whose names were listed in our database also participated after receiving a telephone invitation. Eight visually normal healthy female subjects were also included as controls (Group III) who were age-similar and race-matched for Group II. Informed consent was obtained from all participants. The protocol was approved by an institutional review board of the University of Illinois at Chicago, and conducted in accordance with the principles of the Declaration of Helsinki.
Exclusion criteria for all participants included known optic nerve diseases or anomalies (optic neuropathy, optic disc drusen, optic pit, or coloboma), known retinal diseases (diabetic retinopathy, retinitis pigmentosa, age-related macular degeneration, and ocular occlusive vascular diseases), uveitis, glaucoma, or glaucoma suspects. We also excluded individuals with a diagnosis of diabetes mellitus, intraocular pressure (IOP) higher than 20 mmHg or an earlier history of ocular hypertension, refractive error of more than ±6 D sphere or ±3 D cylinder, earlier intraocular or refractive surgery, and media opacity that precluded a high-quality OCT examination. The control group (Group III) did not have a history of either chloroquine or hydroxychloroquine exposure.
Data collection included date of birth, gender, race, medical, and ophthalmic history, duration of drug exposure, dosages, current and earlier body weight, as well as ocular symptoms (if present). All participants underwent a comprehensive ocular examination, including best-corrected visual acuity (BCVA) using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart (The Lighthouse, Long Island City, NY, USA), slit-lamp examination, IOP measurement with Goldmann applanation tonometry, detailed fundus examination, and colour vision test using Ishihara pseudoisochromatic plates (Kanehara Shuppan Co., Ltd, Tokyo, Japan). The patients in Groups I and II underwent visual field test using the Humphrey 10-2 programme (Zeiss Humphrey Systems, Dublin, CA, USA). Multifocal electroretinography (mfERG) (VERIS, Electro-Diagnostic Imaging, San Mateo, CA, USA) was conducted on the patients in Group II. The protocol for mfERG testing was described in an earlier study.13
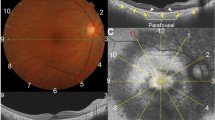
Sd-OCT imaging was carried out on all participants using Optovue technology (RTVue Model-RT100 version 3.5; Optovue Inc., Fremont, CA, USA). The instrument provided internal fixation. The NHM4 protocol was used for peripapillary RNFL analysis. The GCC protocol was conducted for scan acquisition of a 7 mm by 7 mm area within the posterior pole, centering at 1 mm temporal to the foveal centre. It provided automated numerical thickness measurements of the inner, outer, and full-thickness retina from the superior and inferior hemispheres of the posterior pole. The scans had a depth resolution of 3 μm per pixel and spatial resolution of 7.5 μm per pixel.
In this study, we evaluated the peripapillary RNFL thickness using customized and colour-coded normative data available in the OCT programme. In addition, we made quantitative measurements of peripapillary RNFL thickness in each quadrant on Groups I and II, which were compared with our visually normal controls in Group III. Using the GCC protocol, the thickness of the inner, outer, and full-thickness retina in the posterior pole was also quantitatively measured in Groups I and II, compared to that of Group III.
Peripapillary RNFL thickness was measured at a diameter of 3.45 mm around the centre of the optic disc with a total of 2225 A-scans, and was analysed in the colour-coded map using customized software with normative data adjusted for age and disc size. The peripapillary RNFL thickness map is measured as numerical values within each of 16 colour-coded segments circumferentially (four segments in each quadrant). A normal value was shown in green, although an abnormally thin RNFL was encoded yellow and red for values of less than the 5th and 1st percentiles, respectively. Three scans were carried out in each eye to ensure reproducibility. The authors (SP and GAF) reviewed each scan. The scans with poor centration, low signal strength index (SSI), and visibly misaligned segmentation lines were excluded. Additional scans were carried out to provide at least three high-quality scans with acceptable reproducibility of the colour coding in at least two out of three scans in each eye. Numerical RNFL thickness measurements from all segments were tabulated for quantitative analysis without reference to the colour-coded map.
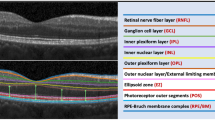
The GCC module automatically measured the average thickness of the inner retina (RNFL+GCL+inner plexiform layer (IPL)), the outer retina (inner nuclear layer (INL) to the apex of RPE), and the full-thickness retina in the 7 × 7 mm area of the posterior pole for each hemisphere (superior and inferior). The numerical thickness values of the inner, outer, and full-thickness retina from each hemisphere were tabulated for the analysis. Accurate segmentation lines were checked in all scans before being accepted for data analysis.
Peripapillary RNFL thickness was analysed using the colour codes in each quadrant: temporal (136–225° for the right and 316–45° for the left), superior (46–135°), nasal (316–45° for the right and 136–225° for the left), and inferior (226–315°). Yellow and red were considered abnormal codes. A thinning of the peripapillary RNFL was determined when at least two of the four segments within a quadrant were observed as abnormal in either one or more of the four quadrants. The reproducibility of an abnormal coding had to occur in at least two out of three high-quality scans. All eyes with non-reproducible data were excluded from the analysis.
For the non-colour code-referenced quantitative analysis, mean RNFL thickness in each eye of each patient in each group was calculated. The values from Groups I and II were analysed and compared to those of Group III using a Student's t-test. A probability value of less than 0.05 was considered statistically significant.
The thickness measurements for the inner, outer, and full-thickness retina were collected and tabulated in each group for statistical analysis using data from the GCC protocol. The mean thickness values between superior and inferior hemispheres in Groups I and II were calculated and compared to those in Group III using the Student's t-test..
Results
Demographic characteristics
This study included four patients in Group I, eight patients in Group II, and eight visually normal controls in Group III (Table 1). Both eyes were studied. All participants were female with the median age of 55.8 years (range: 34–70). The majority of study subjects were Caucasian, except two each in Groups II and III who were African American. The mean age in Groups I, II, and III were 57.6±8.0, 54.9±11.0 and 53.7±10.5 years, respectively. There were no statistically significant differences in age among groups (P=0.827). Of the twelve patients with a history of chloroquine or hydroxychloroquine exposure, seven patients were diagnosed with SLE, three had RA, one had discoid lupus, and one had juvenile idiopathic arthritis (JIA).
All of those in Groups I and II were exposed to hydroxychloroquine, except one patient in Group I who used chloroquine. Duration of exposure ranged from 5 to 12 years (median: 7.5 years) in Group I, and from 6 to 35 years (median: 10 years) in Group II. Maximum daily doses were listed in both absolute numbers and on a by-weight basis. Maximum daily doses, as shown in Table 1, were generally similar to the average doses that the patients had used, except in patient no.1 who switched from 5.87 to 8.80 mg/kg/day only three months before the diagnosis of retinal toxicity. All patients in Group I were diagnosed with typical retinal toxicity and had discontinued therapy. Only one patient in Group II had discontinued medication because of borderline renal insufficiency. Median accumulative doses for Groups I and II were 875 (range: 730–1750) and 1651 g (range: 792–2628), respectively.
The patients in Group I originally presented with visual field defects or central visual complaints. Three patients had a bilateral bull's eye maculopathy without any clinically significant extramacular involvement. One patient (no. 4) with a history of chloroquine exposure retained good central visual function, but presented with inferonasal retinal atrophy compatible with a superotemporal visual field defect on Goldmann perimetry. Humphrey 10-2 visual field testing was normal in all Group II patients (Table 2). Corneal verticillata changes were not seen in any of the study subjects. IOP ranged from 11 to 18 mmHg. All patients in Groups II and III had normal colour vision screening on Ishihara pseudoisochromatic test plates, whereas five of six eyes with a bull's eye maculopathy in Group I showed significant colour vision deficiency. All eight patients in Group II underwent mfERG testing. Reliable testing results were not obtained in two patients (nos. 10 and 12) because of technical difficulties. Five of the six remaining patients had normal mfERG findings throughout the six rings of measurement. One patient (no. 9) had reduction of b-wave amplitudes in two out of six rings in the right eye and five out of six rings in the left eye, compared with a visually normal age-similar population.
Colour code-referenced analysis of peripapillary RNFL thickness
Seven (87.5%) of eight eyes in Group I showed peripapillary RNFL thinning using our criteria in at least one quadrant, whereas none of those in Groups II and III did so. The presence of thinning was not necessarily symmetrical between the two eyes (Table 3). The peripapillary RNFL thinning in the temporal quadrant was seen in three (50%) of six eyes with a bull's eye maculopathy (nos. 1–3). Patient no. 3 presented with asymmetric functional involvement with relatively spared visual acuity, central visual field, and colour vision in the left eye, in which peripapillary RNFL thinning was not detected by our criteria. By observing all eyes in Group I, the peripapillary RNFL thinning was most commonly seen in the nasal and temporal quadrants (37.5% of eyes, each).
Non-colour code-referenced quantitative peripapillary RNFL analysis
Table 4 shows mean thickness of the peripapillary RNFL in each quadrant. By comparing Groups I–III, there were statistically significant differences of thickness in the superior (P=0.037), nasal (P<0.001), and inferior quadrant (P<0.001). There was statistically significant difference only in the nasal quadrant (P=0.026) when comparing between Groups II and III.
Quantitative retinal segmentation analysis
Table 5 shows mean thickness of the inner, outer, and full-thickness retina in the posterior pole of each group using the GCC protocol along with automated numerical thickness analysis available in the OCT instrument. The thickness measurements in Group I were significantly less than those in Group III. (P<0.001 for the inner, outer, or full-thickness retinal measurements). Interestingly, comparative analysis between Groups II and III showed that there was a statistically significant difference in the mean inner retinal thickness (P<0.001), but no significant differences were detected for the outer (P=0.971), or the full-thickness retina (P=0.082).
Discussion
The occurrence of potentially severe ocular side effects from hydroxychloroquine or chloroquine are likely somewhat minimized by the heightened awareness of ophthalmologists and rheumatologists. Recent reports show a low prevalence rate of 0.082–0.5%14 for retinal toxicity in patients with long-term exposure to hydroxychloroquine. Nevertheless, our study shows that, using OCT measurements, defects in the inner retina may be occurring even before the ascertainment of defects at the level of the RPE or photoreceptor cell layer. Our findings also document that once fundoscopic changes of the RPE are apparent, patients are already likely to have thinning of their inner retina including ganglion cells and the RNFL.
Quantitative measurements of the peripapillary RNFL thickness in patients taking chloroquine were previously performed by Bonanomi et al15 using scanning laser polarimetry (GDx nerve fibre analyser). The authors found that RNFL measurements in patients using long-term chloroquine, particularly with high daily doses, were significantly thinner than those of healthy controls in all quadrants. However, the interpretation was limited by using a fixed corneal compensator, which may not eliminate the corneal birefringence potentially impacting RNFL measurement.
In our study, significant peripapillary RNFL thinning was seen in 87.5% of the eyes with retinal lesions compatible with drug toxicity. By using our OCT criteria, none of patients who had long-term exposure to hydroxychloroquine without specific fundus changes showed peripapillary RNFL thinning. This might imply that peripapillary RNFL analysis may not be sensitive to detect changes from drug exposure, or that peripapillary RNFL defects are not present in the absence of fundus changes. However, OCT testing may be a useful tool for establishing the presence of RNFL defects in some patients who have history of chronic use of hydroxychloroquine or chloroquine.
Reductions in peripapillary RNFL thickness were more prominent in the nasal and temporal quadrants in our cohort of patients in Group I on the basis of the colour-coded analysis. This is possibly due to the normally thicker RNFL in the superior and inferior quadrants, and therefore, a defect may be less readily detectable. Patients in Group II had a significantly thinner peripapillary RNLF selectively in the nasal quadrant when compared with controls. Future study of RNFL thickness in the nasal peripapillary region may be useful to detect early changes in the absence of clinically observed retinal signs from drug toxicity, which usually reflect an abnormality of the RPE in the macular region.
Although thinning of the inner retinal layer was observed in our patients without ophthalmoscopically apparent lesions, measurement of the peripapillary RNFL did not disclose evidence of RNFL thinning. We postulate that this may be related to an accentuation or relative prevalence for macular changes to occur in patients who experience drug toxicity from hydroxychloroquine or chloroquine. Peripapillary axonal thinning might not be measurable because of a substantial number of axons that originate from non-macular regions, which contribute substantially to the overall RNFL thickness as measured in the peripapillary region.
We considered that there is a hypothetical possibility that rheumatologic disease itself may possibly have an impact on retinal structural changes. However, we excluded any patients with retinal changes, which may occur in rheumatologic diseases, including vascular sheathing, cotton wool spots, or intraretinal haemorrhages. Further, we recognize that an ideal control group would have been patients, with the same rheumatologic diagnoses for a similar duration, who did not have a history of exposure to hydroxychloroquine or chloroquine.
Our study in the posterior pole of the retina using the OCT GCC protocol showed that the inner retina may initially be primarily affected in the course of drug toxicity with either hydroxychloroquine or chloroquine. This is compatible with earlier reports in animal studies.8, 12 Use of this procedure for longitudinal monitoring of the inner retina in such patients could provide a means for earlier detection of anatomic changes that may occur with the use of these drugs. This, in turn, might prevent future visual loss, which could occur with continued treatment.
References
Bernstein HN . Chloroquine ocular toxicity. Surv Ophthalmol 1967; 12: 415–447.
Levy GD, Munz SJ, Paschal J, Cohen HB, Pince KJ, Peterson T . Incidence of hydroxychloroquine retinopathy in 1207 patients in a large multicenter outpatient practice. Arthritis Rheum 1997; 40: 1482–1486.
Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF . Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology 2002; 109: 1377–1382.
Lowes M . Peripheral visual field restriction in chloroquine retinopathy. Report of a case. Acta Ophthalmol (Copenh) 1976; 54: 819–826.
Butler I . Retinopathy Following the use of chloroquine and allied substances. Ophthalmologica 1965; 149: 204–208.
Duncker G, Schmiederer M, Bredehorn T . Chloroquine-induced lipidosis in the rat retina: a functional and morphological study. Ophthalmologica 1995; 209: 79–83.
Duncker G, Bredehorn T . Chloroquine-induced lipidosis in the rat retina: functional and morphological changes after withdrawal of the drug. Graefes Arch Clin Exp Ophthalmol 1996; 234: 378–381.
Rosenthal AR, Kolb H, Bergsma D, Huxsoll D, Hopkins JL . Chloroquine retinopathy in the rhesus monkey. Invest Ophthalmol Vis Sci 1978; 17: 1158–1175.
Gonasun LM, Potts AM . In vitro inhibition of protein synthesis in the retinal pigment epithelium by chloroquine. Invest Ophthalmol 1974; 13: 107–115.
Ivanina TA, Sakina NL, Lebedeva MN, Borovjagin VL . A study of the mechanisms of chloroquine retinopathy. II. Chloroquine effect on protein synthesis of retina. Ophthalmic Res 1989; 21: 272–277.
Ivanina TA, Sakina NL, Lebedeva MN, Borovyagin VL . A study of the mechanisms of chloroquine retinopathy. I. Chloroquine effect on lipid peroxidation of retina. Ophthalmic Res 1989; 21: 216–220.
Hallberg A, Naeser P, Andersson A . Effects of long-term chloroquine exposure on the phospholipid metabolism in retina and pigment epithelium of the mouse. Acta Ophthalmol (Copenh) 1990; 68: 125–130.
Vajaranant TS, Seiple W, Szlyk JP, Fishman GA . Detection using the multifocal electroretinogram of mosaic retinal dysfunction in carriers of X-linked retinitis pigmentosa. Ophthalmology 2002; 109: 560–568.
Mavrikakis I, Sfikakis PP, Mavrikakis E, Rougas K, Nikolaou A, Kostopoulos C et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology 2003; 110: 1321–1326.
Bonanomi MT, Dantas NC, Medeiros FA . Retinal nerve fibre layer thickness measurements in patients using chloroquine. Clin Experiment Ophthalmol 2006; 34: 130–136.
Acknowledgements
This study was supported by the Foundation Fighting Blindness, Owings Mills, MD, USA; by a grant from Grant Healthcare Foundation, Chicago, IL, USA; NIH core Grant EY01792; and an unrestricted departmental grant from Research to Prevent Blindness.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no financial or proprietary interest in any of the products or techniques mentioned in this study
Rights and permissions
About this article
Cite this article
Pasadhika, S., Fishman, G. Effects of chronic exposure to hydroxychloroquine or chloroquine on inner retinal structures. Eye 24, 340–346 (2010). https://doi.org/10.1038/eye.2009.65
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.65
Keywords
This article is cited by
-
Outcomes of screening for hydroxychloroquine retinopathy at the Manchester Royal Eye Hospital: 2 years’ audit
Eye (2023)
-
Hydroxychloroquine Causes Early Inner Retinal Toxicity and Affects Autophagosome–Lysosomal Pathway and Sphingolipid Metabolism in the Retina
Molecular Neurobiology (2022)
-
Hydroxychloroquine retinopathy — implications of research advances for rheumatology care
Nature Reviews Rheumatology (2018)
-
Early morpho-functional changes in patients treated with hydroxychloroquine: a prospective cohort study
Graefe's Archive for Clinical and Experimental Ophthalmology (2018)
-
Macular ganglion cell–inner plexiform layer thickness for detection of early retinal toxicity of hydroxychloroquine
International Ophthalmology (2018)