Abstract
Purpose
To compare the cytotoxicity of different fluoroquinolones (FQs) towards human corneal epithelial cells (HCECs).
Methods
HCECs were incubated with FQs (norfloxacin, ciprofloxacin, ofloxacin, levofloxacin, moxifloxacin, and gatifloxacin), both as commercial ophthalmic formulations and as unpreserved solutions. Cells incubated in different formulations of gentamicin, cefazolin, and benzalkonium chloride (BAC) were also compared. A cell viability assay, using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay, was used to evaluate the drug effects on cell viability after five incubation times (30 min, 1 h, 4 h, 8 h, and 24 h). Transepithelial electrical resistance (TEER) was measured with a voltohmmeter to help understand changes in paracellular permeability at five time points (4 h, 8 h, 12 h, 24 h, and 48 h). Cell morphology was observed with an inverted fluorescence microscope, with multiple stage position and in time-lapse mode.
Results
The preserving solutions and BAC at concentrations above 0.005% significantly decreased cell viability, when assayed by MTS. Increased paracellular permeability and decreased membrane integrity were also observed by TEER measurements and inverted fluorescence microscopy. Ofloxacin and levofloxacin were both free of preservatives and showed the least cytotoxicity towards HCECs in commercial FQ eye drops.
Conclusions
The cytotoxicity observed with FQ eye drops seems to be caused mainly by the preservative, which induced a significant decrease in membrane integrity and increased paracellular permeability. We found the new generation of FQs (moxifloxacin and gatifloxacin) no less cytotoxic towards HCECs than the old generation ones.
Similar content being viewed by others
Introduction
Bacterial keratitis is a common ocular infection and a leading cause of ocular morbidity and blindness worldwide. However, with appropriate antibiotics selected on the basis of diagnostic corneal smears and cultures, around 72% of infections could be successfully treated topically.1 Fluoroquinolones (FQs) were derived from the non-fluorinated drug nalidixic acid, which was developed during the early 1960 s. FQs are potent antimicrobial agents with a broad antibacterial spectrum and are suitable for monotherapy. They act rapidly by inhibiting bacterial DNA gyrase and topoisomerase IV, which are selective for bacterial cells.2 With different modifications to the basic molecular ‘4-quinolone’ skeleton, several generations of FQs have been developed.3, 4 The hallmark of fourth-generation FQs is the 8-methoxy group (-OCH3), which improves activity against Gram-positive pathogens, whereas retaining the potency of the third-generation agents against Gram-negative pathogens.5 FQs are nowadays widely used in clinical practice to treat ocular infections.
In treating bacterial keratitis, both the drug efficacy and cytotoxicity should be considered when choosing antibiotics. Fourth-generation FQs (moxifloxacin and gatifloxacin) were introduced to ophthalmology to counteract the emerging resistance to third-generation ones (ciprofloxacin, ofloxacin, and levofloxacin).6, 7, 8 The successful treatment of mycobacterial keratitis with fourth-generation FQs has also been reported.9, 10, 11 However, bacterial keratitis resistant to fourth-generation FQs has been found12 and the prophylactic usage of moxifloxacin and gatifloxacin did not significantly lower the endophthalmitis rate after uncomplicated cataract surgery either.13 Although the superiority of clinical efficacy between different FQs is still under evaluation,14, 15, 16, 17, 18, 19, 20 controversial results exist that compare the toxicity of FQs towards the corneal epithelial cells.19, 21, 22, 23 The ocular and systemic safety profile of newer FQs is generally considered comparable with those of other FQ antimicrobial agents, with a low recognized risk of quinolone-related toxicity.24, 25, 26, 27 However, Walter et al28 reported two cases of severe sterile corneal ulcers after the topical application of moxifloxacin, and the drug was suspected of interfering with corneal healing.
Several factors may contribute to the cytotoxicity of FQ eye drops. Besides the toxicity from the antibiotics itself, other components of the formulation including osmolarity, pH, and the presence of the preservatives benzalkonium chloride (BAC) may all contribute. The purpose of our study is to compare the cytotoxicity between different FQs and other commonly used antibiotics, including commercial gentamicin eye drops (0.3%), unpreserved fortified antibiotics (1.4% gentamicin, and 5% cefazolin) and BAC (0.001, 0.005, and 0.01%). The results could serve as a reference for the clinical application of FQs, as well as help clarify the source of cytotoxicity within these drugs.
Materials and methods
Test materials
We used both the raw materials (standard powders) and commercially available ophthalmic solutions of FQs as testing materials. The standard powders were sourced as follows: norfloxacin (Sigma-Aldrich Inc., Milwaukee, WI, USA), ciprofloxacin (Bayer AG, Leverkusen, Germany), ofloxacin (Daiichi Pharmaceutical Co., Tokyo, Japan), levofloxacin (Daiichi Pharmaceutical Co.), moxifloxacin (Bayer AG), and gatifloxacin (Kyorin Pharmaceutical Co., Tokyo, Japan). Ophthalmic solutions of the following were used in this study: norfloxacin (0.3%, Baccidal; Kyorin Pharmaceutical Co.), ciprofloxacin (0.3%, Ciloxan; Alcon Laboratories Inc., Puurs, Belgium), ofloxacin (0.3%, Oflovid; Santen Pharmaceutical Co., Osaka, Japan), levofloxacin (0.5%, Cravit; Santen Pharmaceutical Co.), moxifloxacin (0.5%, Vigamox; Alcon Laboratories Inc., Fort Worth, TX, USA), and gatifloxacin (0.3%, Zymar; Allergan Inc., Irvine, CA, USA). Also tested and compared against the FQs were gentamicin ophthalmic solution (0.3%, Garamycin; Schering-Plough, Brussels, Belgium), fortified antibiotic solution prepared from parental gentamicin (U-Gencin 40 mg/ml, U-Liang Pharmaceutical Co., Jhongli City, Taiwan), and cefazolin (Cefamezin 1.0 g/vial; Taiwan Biotech Co., Taoyuan, Taiwan) and BAC (Sigma-Aldrich Inc.).
Preparation of test solutions
The standard powders of norfloxacin, ciprofloxacin, ofloxacin, levofloxacin, and gatifloxacin were dissolved in distilled water to a stock concentration of 3% (w/v). A 1.8% moxifloxacin solution in distilled water was prepared. FQ stock solutions were diluted with medium (pH ∼7.3) to 0.3% (market concentration) immediately before cell treatments. The pH was adjusted by 2N HCl titration to 6.5–7.0, except for norfloxacin (5.0–5.5) and ciprofloxacin (4.5–4.8). Preservative-free parental gentamicin was diluted to 1.4% with distilled water. Cefazolin powder was dissolved in distilled water and diluted to make a 5% solution (pH ∼7) with distilled water before use. A stock solution of 0.2% BAC (pH 6.9) prepared in distilled water was further diluted to 0.01, 0.005, and 0.001% with distilled water and 0.0005% with culture medium before cell treatments. The antibiotics prepared herein did not contain preservatives or pharmaceutical excipients. Although not proven, the observed effects may mostly be attributed to the test solutions per se.
The ophthalmic solutions of gatifloxacin (Zymar) (pH 6.0) and gentamicin (Garamycin) (pH 7.0) contained 0.005% BAC. Ciprofloxacin solution (Ciloxan) (pH 4.5) contained 0.006% BAC. Norfloxacin (Baccidal) (pH 5.0–5.6), ofloxacin (Oflovid) (pH 6.0–7.0), levofloxacin (Cravit) (pH 6.2–6.8), and moxifloxacin (Vigamox) (pH 6.8) were BAC-free preparations. The osmolarities were all within 0.85–1.15 times that of normal saline. In transepithelial electrical resistance (TEER) measurements, we diluted these commercial drugs to 1/10 concentration with culture medium.
The medium used for test solutions preparation did not contain serum or antibiotics.
Cell culture
A human corneal epithelial cell line (HCECs) (ATCC CRL, 11515) was used in this study. The cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM; Gibco Laboratories, Grand Island, NY, USA) supplemented with 10% foetal bovine serum, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 0.125 μg/ml amphotericin B at 37°C in a moist atmosphere with 5% CO2. The culture medium was changed every 2 days.
MTS assay
For viability studies, cells were seeded in 96-well plates (2 × 104 cells/well), incubated in DMEM and supplemented as described above for 24 h. Once a confluent cell layer was obtained, the culture medium was withdrawn and the cells were then incubated for the further 18 h with fresh medium without serum and antibiotics supplement. Afterwards, the media were removed and 100 μl of the test materials were added in various concentrations (market ophthalmic solution of FQs and gentamicin; preservative-free fortified antibiotics of 1.4% gentamicin and 5% cefazolin; 0.3% solutions of FQ powders and BAC solutions of 0.01, 0.005, and 0.001%). The MTS assay (CellTiter 96 AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI, USA) was performed at five incubation time points (30 min, 1 h, 4 h, 8 h, and 24 h). The test materials were removed and 20 μl of MTS reagent along with 100 μl of culture medium (without serum and antibiotics) were added to each well. HCECs were further incubated at 37°C for 3 h. Absorbance measurements were read at 490 nm using a fluorescence/absorbance 96-well plate reader afterwards. Negative control groups consisted of cells in media (without serum and antibiotics) without the test materials added (pH=7.3). Groups of media, pH adjusted to 5.0 (without serum, antibiotics or test materials added), were processed identically and incubated alongside the treated groups. Three independent experiments (n=4 per group) were done. Cell viability was expressed as a percentage of the negative control group.
To distinguish the toxicity from water and ensure the adequacy of nutrient for cell growth, we compared HCECs cultured in media without test materials (negative control) and those in distilled water (vehicle control). MTS assay were performed at 1 h, 4 h, 8 h, 12 h, 16 h, 20 h, and 24 h between these two groups.
Transepithelial electrical resistance
Transepithelial electrical resistance was measured with a voltohmmeter (Millicell-ERS, Millipore, Billerica, MA, USA) to help monitor changes in paracellular permeability. Cells (4.55 × 105 cells/insert) were seeded on transwell polycarbonate inserts (12-well format, 12 mm insert diameter) with a mean pore size of 0.4 μm (Transwell, Corning, Lowell, MA, USA) after being coated with fibronectin and serum-free DMEM (1 : 10). After 3 days, the cells reached confluency (raw resistance above 140 Ω cm2) and the medium was substituted with serum- and antibiotics-free medium for further culture for 24 h. When the raw resistance reached 200 Ω cm2, the medium was replaced with 2 ml (0.5 ml within insert and 1.5 ml outside the insert each well) of 0.0005% BAC (1/10 of the concentration present in market ophthalmic solutions), six commercial FQ and gentamicin drops at 1/10 of market concentration, for better observing the changes of paracelluar permeability. Cells grown in culture medium only (without serum and antibiotics) served as control group (pH=7.3). Cells in media pH adjusted to 5.0 without any supplement were processed alongside the treated groups. Blanks (inserts without cells, containing media only) were used to determine background values (raw resistance around 110 Ω cm2). TEER was measured at 4 h, 8 h, 12 h, 24 h and 48 h. Three independent experiments (n=3 per group per experiment) were done. The background TEER of blank transwell filters was subtracted from the TEER of the cell monolayer. TEER was calculated from the measured resistance and normalized by the area of the monolayer (ohms per cm2). Values were expressed as percentage of the control groups at each time point.
Inverted fluorescence microscopy
Cell morphology was observed and recorded by inverted fluorescence microscopy (Axiovert 200 M, Zeiss, Thornwood, NY, USA). Cells were seeded in 24-well plate (8 × 104/well) incubated in DMEM, supplemented as described above for 24 h. The medium was withdrawn after the cells were confluent, and serum- and antibiotics-free medium was added for further incubation. After 18 h, the medium was removed and preservative-free 0.3% FQ solutions were added (n=2 for each group). Multiple stage position, time-lapse microscopy was used to record the cell morphology during drug treatments. Images were taken every 10 min over a 16 h period.
Statistical analysis
Values were expressed as the mean percentages of control values±SD from three independent experiments, each in four or three replicates (n=12 in MTS assay and n=9 in TEER). The t-tests were used to compare test group with the control group or with another test group. If normality or the equal variance test was not satisfied, the Mann–Whitney rank-sum test was applied. A P-value <0.05 was considered as statistically significant. All statistical data were analysed between different groups using Kruskal–Wallis one-way analysis of variance on ranks and by pairwise multiple comparison (Dunn's method) (Sigmastat 2.03 SPSS Inc., Chicago, IL, USA). Bonferroni-adjusted P-value was used in multiple groups comparison, a P-value <0.05/n (where n is the number of groups) was considered as statistically significant.
Results
Cytotoxicity on HCECs: MTS assay (Table 1 and Figure 1a)
Commercial FQ and gentamicin ophthalmic solutions
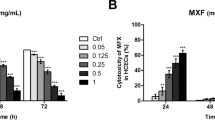
Comparison of cytotoxicity to HCECs from commercial preparations (a) MTS assay (% of control): HCECs incubated with commercial fluoroquinolone solutions, parental fortified gentamicin 1.4% and cefazolin 5%, benzalkonium chloride (0.01, 0.005, and 0.001%) and pH=5 medium, compared with negative control in four time points. (n=12, 4 replicates each of three independent experiments) (b) TEER (% of control), HCECs incubated in 1/10 concentration of commercial fluoroquinolone and gentamicin solutions, 0.0005% benzalkonium chloride and pH=5 medium. After 12 h of incubation, ciprofloxacin induced a significant reduction of TEER compared with the other groups. After 48 h of incubation, TEER of gentamicin, 0.0005% BAC, ciprofloxacin, and gatifloxacin groups were significantly decreased. (n=9, 3 replicates each of three independent experiments) ((NOR) norfloxacin, (CIP) ciprofloxacin, (OFL) ofloxacin, (LEV) levofloxacin, (MOX) moxifloxacin, (GAT) gatifloxacin, (GEN) gentamicin, (Ugen) parental fortified gentamicin, (Cefa) parental fortified cefazolin, (BAC) benzalkonium chloride).
Significant cell toxicity was found in the ciprofloxacin, gentamicin, gatifloxacin (Mann–Whitney rank-sum test, P<0.001), and moxifloxacin groups (Mann–Whitney rank-sum test, P=0.01) compared with the control group after a 30-min incubation. After 4 h, all the experiment groups showed a significant decrease in cell viability except for in levofloxacin and ofloxacin groups, and there was no difference between these two groups (t-test, P=0.399). After 8 h, cells in all groups showed a significantly reduced viability compared with the control cells (Mann–Whitney rank-sum test, P<0.001), with levofloxacin and ofloxacin showing relatively less cytotoxicity.
Commercial FQ and gentamicin ophthalmic solutions compared with preservative-free, parental fortified 1.4% gentamicin and 5% cefazolin
After 1 h, 5% cefazolin did not show significant cytotoxicity compared with ofloxacin and control (t-test, P=0.789 and P=1.00, respectively). The viability of cells treated with 1.4% gentamicin solution was significantly decreased compared with the control cells (Mann–Whitney rank-sum test, P=0.009). However, the cytotoxicity induced by 1.4% gentamicin solution was significantly less than ciprofloxacin, gentamicin, and gatifloxacin ophthalmic solutions (t-test, P<0.001). After 4 h, 1.4% gentamicin and 5% cefazolin showed less cytotoxicity towards HCECs than levofloxacin and ofloxacin (t-test, P<0.001). Even after 8 h treatment, good cell viability was maintained in the 5% cefazolin and 1.4% gentamicin groups.
Commercial FQ and gentamicin ophthalmic solutions compared with BAC
Cells treated with 0.01, 0.005, and 0.001% BAC were incubated simultaneously with the other groups. After 30 min, 0.01 or 0.005% BAC showed significant cytotoxicity towards HCECs compared with the control group (Mann–Whitney rank-sum test, P<0.001). However, 0.005% BAC induced less cytotoxicity than ciprofloxacin, gentamicin (t-test, P<0.001, P=0.002, respectively). The cytotoxicity induced by moxifloxacin was significantly less than that caused by 0.005% BAC at 1 h (t-test, P=0.005). However, the difference was not significant after 4 h (t-test, P=0.482).
Effects of groups of chemical-free medium at pH 5 and of distilled water, compared with negative control (chemical-free medium at pH 7.3)
There were no statistically significant differences in viability between cells incubated with chemical-free medium at pH 5 and at pH 7.3, as measured at five time points (30 min, 1–24 h). For HCECs cultured in media and in distilled water, significant cytotoxicity was found at 12 h (t-test, P=0.031) between these two control groups.
Transepithelial electrical resistance
There were no statistically significant differences among testing groups in terms of TEER changes within the initial 8 h. (Table 2, Figure 1b) After 12 h, ciprofloxacin induced a significant reduction of TEER compared with the other groups. Ciprofloxacin and gentamicin induced significant decreases in TEER after 24 h of treatment, whereas levofloxacin and ofloxacin maintained TEER most of all. After 48 h, TEER measurements of the gentamicin, 0.0005% BAC, ciprofloxacin, and gatifloxacin groups were significantly decreased. Still, ofloxacin and levofloxacin retained the highest TEER (t-test, P=0.114 between two groups). For cells treated with medium adjusted to pH 5, TEER was significantly decreased compared with the control group only after 48 h of incubation (t-test, P=0.012).
Raw materials (standard powder) of FQs: MTS assay
After treating HCECs with pH adjusted, preservative-free 0.3% FQ solutions for 1 h, moxifloxacin and ciprofloxacin showed statistically significant toxicity compared with the control (Mann–Whitney rank-sum test, P<0.001, P=0.005, respectively) (Table 3, Figure 2). After 4 h, the viability of HCECs exposed to moxifloxacin, ciprofloxacin, gatifloxacin, and norfloxacin was significantly decreased (t-test, P<0.001) with respect to the control. After 8 h, moxifloxacin showed the greatest cytotoxicity (t-test, P<0.001) of all the groups except for comparing with ciprofloxacin (Mann–Whitney rank-sum test, P=0.221). HCEC cells treated with ofloxacin showed the best cell viability after 24 h of incubation. (Mann–Whitney rank-sum test, P=0.013 with levofloxacin).
MTS assay (% of control): HCECs incubated with preservative-free 0.3% fluoroquinolone solutions compared with negative control. After 1 h of incubation, moxifloxacin and ciprofloxacin showed statistically significant toxicity. After 8 h of incubation, moxifloxacin showed the greatest cytotoxicity. After 24 h of incubation, HCECs treated with ofloxacin showed the best cell viability after 24 h incubation. (n=12, 4 replicates each of three independent experiments) ((NOR), (CIP), (OFL), (LEV), (MOX), (GAT): same as Figure 1).
Cell morphology: inverted fluorescence microscopy
In a series of microscopy images, we observed that cells incubated with moxifloxacin detached from the culture plate within just 1 h and were completely suspended within 2 h. (Figure 3) A higher proportion of the cells remained viable in the ofloxacin and levofloxacin groups. At the end of the recording, the control group cells remained confluent and kept in cuboid form, which was similar to primary cultured corneal epithelial cells.
HCECs treated with preservative-free 0.3% FQ solutions. The images were taken from inverted fluorescence microscopy with multiple stage position, in time-lapse mode. After 2 h, HCECs treated with moxifloxacin had all detached from the culture well (a). Cells treated with norfloxacin and ciprofloxacin had become elongated in shape (b). ((NOR), (CIP), (OFL), (LEV), (MOX), (GAT): same as Figure 1).
Discussion
The epithelium is the main barrier of the cornea. The level of topical drug penetration varies up to 20-fold, depending on the integrity of the corneal epithelium.29 Owing to the corneal epithelial barrier, the eye is relatively impermeable to microorganisms and other environmental elements. The healing of epithelial defects is also an important indicator that bacterial keratitis has been effectively treated, as well as being a crucial factor that affects visual outcome after refractive surgery. To eradicate the bacteria during infectious keratitis, topical medications should be given at a very high frequency. Therefore, it is important to choose an appropriate topical medication, which has the least cytotoxicity.
In the initial stages of our study, we used the MTS assay to evaluate cell viability after treating cells with commercial ophthalmic solutions. Cell viability decreased dramatically in the ciprofloxacin, gentamicin, and gatifloxacin groups after only 30 min of incubation. The most significant difference in the formulation of these three commercial eye drops from the other FQs is the presence of BAC. BAC is the most frequently added preservatives in ophthalmic solutions. Its cytotoxic effect to corneal and conjunctival cells had been established in earlier studies.30, 31 To further support our studies, we used HCECs cultured in different concentrations of BAC to compare HCECs in FQ and in gentamicin solutions. In the first 30 min, the commercial ciprofloxacin and gentamicin solutions showed greater cytotoxicity than 0.005% BAC alone. However, cell viability in these three groups plus gatifloxacin group showed no difference after 1 h of treatment. We could presume that other factors such as pH and the chemical structures of the drugs aggravated the cytotoxicity besides the 0.005% BAC present in commercial ciprofloxacin and gentamicin solutions.
The stratified corneal epithelium consists of 5–7 layers of cells, with tight junctions (TJs) at the superficial layer.32 Loosening of TJs may be an early change in the epithelial barrier followed by destructive events caused by drug toxicity. Immortalized HCECs grown on filters, using various filter materials and coating procedures, could be used as an in vitro model that mimics the intact cornea.33, 34 A decrease of TEER indicates an increase in paracellular permeability, which represents the disruption of TJs. HCECs died and detached from the wells rapidly after treatment with commercial FQ solutions in the MTS assay. Therefore, we diluted the solutions to 1/10 the concentration to better observe changes in TEER. Similar to the results of the MTS assay, TEER decreased most evidently in the ciprofloxacin and gentamicin groups during the initial stages. After 48 h, those incubations containing BAC gave the lowest TEER values. To elucidate the role of BAC and to compare the cytotoxicity of the distinct chemical characteristics of the different FQs, we used the standard antibiotic powders and the MTS assay for testing. The viability of cells treated with moxifloxacin decreased most rapidly among all the FQs tested in the initial 8 h. Ofloxacin showed less cytotoxicity. Our results suggest that there are differences in the intrinsic cytotoxicity of the various FQs, quite separate from the influence of additives and pH.
The most common fortified antibiotics used to treat bacterial keratitis are 1.4% gentamicin and 5% cefazolin. Some earlier studies compared the cytotoxicity between FQs, ofloxacin and the aminoglycoside, netilmicin.35, 36, 37 They concluded that netilmicin was significantly less toxic than ofloxacin to human keratocytes,37 to intact and mechanically damaged rabbit eyes,36 and to rabbit corneal epithelial cells.35 Mallari et al38 also found an increased risk of corneal perforation after FQ treatment for bacterial keratitis, compared with treatment with fortified antibiotics (cephazolin 5% and tobramycin 1.36%).In our study, preservative-free fortified gentamicin and cefazolin were less cytotoxic to corneal epithelium cells than all the other FQ solutions tested.
Regarding pH, norfloxacin and ciprofloxacin need to be dissolved in a weakly acidic environment (around pH 6.0, 5.0, respectively). From our results, the HCECs cultured in a low pH environment (pH=5) did not show significant cytotoxicity over the control cells (pH=7.3) even after 24 h of incubation. A low pH environment might pose an adverse influence on cell growth and may account in part for the cytotoxic effects of norfloxacin and ciprofloxacin. However, the effect was not significant compared with other factors such as preservatives and chemical drug structures per se. Although our experiments did not show that pH had a significant influence on the viability of HCECs, the more neutral environment in tear film might cause the precipitation of norfloxacin and ciprofloxacin, which may delay epithelial healing of ulcerative keratitis and result in corneal perforation.39, 40, 41
Numerous studies have compared the cytotoxicity of different FQs, but the results remain controversial. Burka et al21 compared the epithelial wound healing after the treatment of photorefractive keratectomy with moxifloxacin (Vigamox) or with gatifloxacin (Zymar), and found that the eye treated with moxifloxacin healed faster. Kovoor et al,22 using confocal microscopy to evaluate corneal epithelium and stromal thickness after treating rabbits with different FQs, found that moxifloxacin induced less damage than other FQs. However, the ofloxacin and levofloxacin they tested contained 0.005% BAC, which was different from the commercial solutions we tested. Another study, using confocal microscopy and ZO-1 staining on rabbit eyes, also showed that moxifloxacin resulted in better corneal epithelial integrity and TJ organization than gatifloxacin did, after a short-term, intensive exposure.23 Kim et al19 found levofloxacin (0.5%, Cravit; Santen Pharmaceutical Co.) was less toxic than moxifloxacin (0.5%, Vigamox; Alcon Laboratories Inc.) to corneal epithelial cells, using the MTT assay and by measuring the rate of wound healing in vitro. The levofloxacin they tested was a preservative-free formulation, the same as the one we used. Matsumoto et al42 used the standard powders of FQs to compare them and found that moxifloxacin was a stronger inhibitor of corneal epithelial cell migration than gatifloxacin at the same concentration, at high dose. They ranked FQs in order of decreasing inhibition of cell proliferation for HCECs as ciprofloxacin= moxifloxacin>gatifloxacin>levofloxacin>ofloxacin= untreated, which is similar to our results. In our study, we used both commercial ophthalmic solutions and standard FQ powders for comparison. We also compared the fortified, parental antibiotics with commercial FQ drops, as well as the preservative BAC and the effects of using a lower pH medium, in the hopes of better clarifying the source of cytotoxicity caused by FQs. The limitation of our study was the in vitro setting and the use of immortalized HCEC line instead of primary cultures of corneal epithelial cells. However, owing to the scarcity of available tissue and restricted lifespan of primary cultures, immortalized cell lines is a practical tool for toxicity screening of ocular drugs with good sensitivity and reliability.43 The results could serve as a reference for clinical application.
In conclusion, our study showed that the main source of cytotoxicity from commercial FQ ophthalmic solutions came from the preservatives. Gentamicin and cefazolin, without any preservatives, were less toxic to the corneal epithelium, even when applied at a fortified concentration. The intrinsic toxicity of FQs towards HCECs still varied, regardless of any preservatives present. In our study, fourth-generation FQs were not less toxic to HCECs than the older generation ones.
References
Fong CF, Tseng CH, Hu FR, Wang IJ, Chen WL, Hou YC . Clinical characteristics of microbial keratitis in a university hospital in Taiwan. Am J Ophthalmol 2004; 137 (2): 329–336.
Smith A, Pennefather PM, Kaye SB, Hart CA . Fluoroquinolones: place in ocular therapy. Drugs 2001; 61 (6): 747–761.
Blondeau JM . A review of clinical trials with fluoroquinolones with an emphasis on new agents. Expert Opin Investig Drugs 2000; 9 (2): 383–413.
Blondeau JM . Fluoroquinolones: mechanism of action, classification, and development of resistance. Surv Ophthalmol 2004; 49 (Suppl 2): S73–S78.
Dong Y, Xu C, Zhao X, Domagala J, Drlica K . Fluoroquinolone action against mycobacteria: effects of C-8 substituents on growth, survival, and resistance. Antimicrob Agents Chemother 1998; 42 (11): 2978–2984.
Goldstein MH, Kowalski RP, Gordon YJ . Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology 1999; 106 (7): 1313–1318.
Chaudhry NA, Flynn Jr HW, Murray TG, Tabandeh H, Mello Jr MO, Miller D . Emerging ciprofloxacin-resistant Pseudomonas aeruginosa. Am J Ophthalmol 1999; 128 (4): 509–510.
Alexandrakis G, Alfonso EC, Miller D . Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 2000; 107 (8): 1497–1502.
Hamam RN, Noureddin B, Salti HI, Haddad R, Khoury JM . Recalcitrant post-LASIK Mycobacterium chelonae keratitis eradicated after the use of fourth-generation fluoroquinolone. Ophthalmology 2006; 113 (6): 950–954.
Sarayba MA, Shamie N, Reiser BJ, Sweet PM, Taban M, Graff JM et al Fluoroquinolone therapy in Mycobacterium chelonae keratitis after lamellar keratectomy. J Cataract Refract Surg 2005; 31 (7): 1396–1402.
Lee SB, Oliver KM, Strube YN, Mohan SK, Slomovic AR . Fourth-generation fluoroquinolones in the treatment of mycobacterial infectious keratitis after laser-assisted in situ keratomileusis surgery. Can J Ophthalmol 2005; 40 (6): 750–753.
Moshirfar M, Mirzaian G, Feiz V, Kang PC . Fourth-generation fluoroquinolone-resistant bacterial keratitis after refractive surgery. J Cataract Refract Surg 2006; 32 (3): 515–518.
Moshirfar M, Feiz V, Vitale AT, Wegelin JA, Basavanthappa S, Wolsey DH . Endophthalmitis after uncomplicated cataract surgery with the use of fourth-generation fluoroquinolones: a retrospective observational case series. Ophthalmology 2007; 114 (4): 686–691.
Thibodeaux BA, Dajcs JJ, Caballero AR, Marquart ME, Girgis DO, O'Callaghan RJ . Quantitative comparison of fluoroquinolone therapies of experimental Gram-negative bacterial keratitis. Curr Eye Res 2004; 28 (5): 337–342.
Constantinou M, Daniell M, Snibson GR, Vu HT, Taylor HR . Clinical efficacy of moxifloxacin in the treatment of bacterial keratitis: a randomized clinical trial. Ophthalmology 2007; 114 (9): 1622–1629.
Dajcs JJ, Thibodeaux BA, Marquart ME, Girgis DO, Traidej M, O'Callaghan RJ . Effectiveness of ciprofloxacin, levofloxacin, or moxifloxacin for treatment of experimental Staphylococcus aureus keratitis. Antimicrob Agents Chemother 2004; 48 (6): 1948–1952.
Aliprandis E, Ciralsky J, Lai H, Herling I, Katz HR . Comparative efficacy of topical moxifloxacin versus ciprofloxacin and vancomycin in the treatment of Paeruginosa and ciprofloxacin-resistant MRSA keratitis in rabbits. Cornea 2005; 24 (2): 201–205.
Jensen H, Zerouala C, Carrier M, Short B . Comparison of ophthalmic gatifloxacin 0.3% and ciprofloxacin 0.3% in healing of corneal ulcers associated with Pseudomonas aeruginosa-induced ulcerative keratitis in rabbits. J Ocul Pharmacol Ther 2005; 21 (1): 36–43.
Kim SY, Lim JA, Choi JS, Choi EC, Joo CK . Comparison of antibiotic effect and corneal epithelial toxicity of levofloxacin and moxifloxacin in vitro. Cornea 2007; 26 (6): 720–725.
Kowalski RP, Dhaliwal DK, Karenchak LM, Romanowski EG, Mah FS, Ritterband DC et al Gatifloxacin and moxifloxacin: an in vitro susceptibility comparison to levofloxacin, ciprofloxacin, and ofloxacin using bacterial keratitis isolates. Am J Ophthalmol 2003; 136 (3): 500–505.
Burka JM, Bower KS, Vanroekel RC, Stutzman RD, Kuzmowych CP, Howard RS . The effect of fourth-generation fluoroquinolones gatifloxacin and moxifloxacin on epithelial healing following photorefractive keratectomy. Am J Ophthalmol 2005; 140 (1): 83–87.
Kovoor TA, Kim AS, McCulley JP, Cavanagh HD, Jester JV, Bugde AC et al Evaluation of the corneal effects of topical ophthalmic fluoroquinolones using in vivo confocal microscopy. Eye Contact Lens 2004; 30 (2): 90–94.
Ly LT, Cavanagh HD, Petroll WM . Confocal assessment of the effects of fourth-generation fluoroquinolones on the cornea. Eye Contact Lens 2006; 32 (4): 161–165.
Williams KK, Shepard AR, Rice RL, McCartney MD, Wax MB, Hiddemen JW . Corneal wound healing in New Zealand white rabbits following anterior keratectomy and treatment with moxifloxacin ophthalmic solution 0.5% or gatifloxacin ophthalmic solution 0.3%. J Ocul Pharmacol Ther 2007; 23 (6): 517–525.
Barequet IS, Habot-Wilner Z, Lavinsky F, Ziv H, Belkin M, Rosner M . Effect of fourth-generation fluoroquinolones on the healing rate of corneal erosions in an animal model. Cornea 2007; 26 (5): 606–609.
Herrygers LA, Noecker RJ, Lane LC, Levine JM . Comparison of corneal surface effects of gatifloxacin and moxifloxacin using intensive and prolonged dosing protocols. Cornea 2005; 24 (1): 66–71.
McGee DH, Holt WF, Kastner PR, Rice RL . Safety of moxifloxacin as shown in animal and in vitro studies. Surv Ophthalmol 2005; 50 (Suppl 1): S46–S54.
Walter K, Tyler ME . Severe corneal toxicity after topical fluoroquinolone therapy: report of two cases. Cornea 2006; 25 (7): 855–857.
Fukuda M, Inoue A, Sasaki K, Takahashi N . The effect of the corneal epithelium on the intraocular penetration of fluoroquinolone ophthalmic solution. Jpn J Ophthalmol 2004; 48 (2): 93–96.
Cha SH, Lee JS, Oum BS, Kim CD . Corneal epithelial cellular dysfunction from benzalkonium chloride (BAC) in vitro. Clin Experiment Ophthalmol 2004; 32 (2): 180–184.
Dutot M, Pouzaud F, Larosche I, Brignole-Baudouin F, Warnet JM, Rat P . Fluoroquinolone eye drop-induced cytotoxicity: role of preservative in P2X7 cell death receptor activation and apoptosis. Invest Ophthalmol Vis Sci 2006; 47 (7): 2812–2819.
Sugrue SP, Zieske JD . ZO1 in corneal epithelium: association to the zonula occludens and adherens junctions. Exp Eye Res 1997; 64 (1): 11–20.
Yi X, Wang Y, Yu FS . Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Invest Ophthalmol Vis Sci 2000; 41 (13): 4093–4100.
Toropainen E, Ranta VP, Talvitie A, Suhonen P, Urtti A . Culture model of human corneal epithelium for prediction of ocular drug absorption. Invest Ophthalmol Vis Sci 2001; 42 (12): 2942–2948.
Scuderi AC, Paladino GM, Marino C, Trombetta F . In vitro toxicity of netilmicin and ofloxacin on corneal epithelial cells. Cornea 2003; 22 (5): 468–472.
Marino C, Paladino GM, Scuderi AC, Trombetta F, Mugridge K, Enea V . In vivo toxicity of netilmicin and ofloxacin on intact and mechanically damaged eyes of rabbit. Cornea 2005; 24 (6): 710–716.
Leonardi A, Papa V, Fregona I, Russo P, De Franchis G, Milazzo G . In vitro effects of fluoroquinolone and aminoglycoside antibiotics on human keratocytes. Cornea 2006; 25 (1): 85–90.
Mallari PL, McCarty DJ, Daniell M, Taylor H . Increased incidence of corneal perforation after topical fluoroquinolone treatment for microbial keratitis. Am J Ophthalmol 2001; 131 (1): 131–133.
Wilhelmus KR, Abshire RL . Corneal ciprofloxacin precipitation during bacterial keratitis. Am J Ophthalmol 2003; 136 (6): 1032–1037.
Eiferman RA, Snyder JP, Nordquist RE . Ciprofloxacin microprecipitates and macroprecipitates in the human corneal epithelium. J Cataract Refract Surg 2001; 27 (10): 1701–1702.
Konishi M, Yamada M, Mashima Y . Corneal ulcer associated with deposits of norfloxacin. Am J Ophthalmol 1998; 125 (2): 258–260.
Matsumoto S, Way W, Tarlo K, Short B . Comparative toxicity of fluoroquinolone antibiotics on corneal cells in vitro. Cornea 2006; 25 (2): S1–S7.
Saarinen-Savolainen P, Jarvinen T, Araki-Sasaki K, Watanabe H, Urtti A . Evaluation of cytotoxicity of various ophthalmic drugs, eye drop excipients and cyclodextrins in an immortalized human corneal epithelial cell line. Pharm Res 1998; 15 (8): 1275–1280.
Author information
Authors and Affiliations
Corresponding author
Additional information
Proprietary interest: None.
Presented at the Asia ARVO meeting, 2–5 March 2007, Singapore.
Supported by the Department of Medical Research at the National Taiwan University Hospital
Rights and permissions
About this article
Cite this article
Tsai, TH., Chen, WL. & Hu, FR. Comparison of fluoroquinolones: cytotoxicity on human corneal epithelial cells. Eye 24, 909–917 (2010). https://doi.org/10.1038/eye.2009.179
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.179
Keywords
This article is cited by
-
Silver ciprofloxacin (CIPAG): a successful combination of chemically modified antibiotic in inorganic–organic hybrid
JBIC Journal of Biological Inorganic Chemistry (2018)