Abstract
Purpose
To determine the proportion of patients presenting with thromboembolic central retinal artery occlusion (CRAO) who had undiagnosed vascular risk factors amenable to modification.
Methods
A retrospective audit of consecutive patients with non-arteritic/thromboembolic CRAO presenting between 1997 and 2008 in a single tertiary teaching hospital.
Results
Thirty-three patients with non-arteritic CRAO were identified. Twenty-one patients (64%) had at least one new vascular risk factor found after the retinal occlusive event, with hyperlipidemia being the most common undiagnosed vascular risk factor at the time of the sentinel CRAO event (36%). Nine patients (27%) had newly diagnosed hypertension or previous diagnosis of hypertension but not optimally controlled. To better control their vascular risk factors 18 patients (54%) were given a new or altered medication. Nine patients had more than 50% of ipsilateral carotid stenosis ; six of these proceeded with carotid endarterectomy or stenting. One patient had significant new echocardiogram finding. Systemic ischaemic event post CRAO occurred in two patients with stroke and acute coronary syndrome.
Conclusions
Patients presenting with CRAO often have a previously undiagnosed vascular risk factor that may be amenable to medical or surgical treatment. As this population is at a high risk of secondary ischaemic events, risk factor modification is prudent.
Similar content being viewed by others
Introduction
Central retinal artery occlusion (CRAO) is defined as an occlusion of the central retinal artery usually by a fibrin-platelet thrombus or embolus with resultant reduced perfusion to the retina and painless visual loss that is frequently irreversible.1, 2
CRAO by virtue of its pathogenesis shares important risk factors with other vascular diseases such as ischaemic heart disease and cerebrovascular disease.3 It is known from the study of heart disease and stroke, that following the occurrence of a sentinel vascular event, the patient is more likely to develop subsequent vascular events.4, 5 Similarly, CRAO may also be the harbinger of a more serious vascular event.6, 7 The current management of CRAO is aimed at secondary prevention to prevent another ischemic event.
The aim of this paper is to quantify the pre-existing vascular risk factors in a cohort of patients with CRAO and determine the undiagnosed vascular risk factors amenable to modification following the CRAO event.
Methods
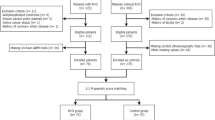
An audit of a cohort of CRAO patients admitted to the Flinders Medical Centre between January 1997 and September 2008 was conducted. The diagnosis of CRAO was based on the initial ophthalmologist's clinical diagnosis with documented visual disturbance due to retinal artery occlusion with evidence of a cherry red spot and attenuation of blood vessels on funduscopic examination. Arteritic CRAO was excluded either clinically based on the presence of symptoms and the presence of an elevated ESR or where possible on temporal artery biopsy.
A single observer abstracted data from the case notes. Data that were collected included demographic details, the previous medical history with a particular emphasis on a subject's previous risk factors such as hypertension, diabetes, hypercholesterolemia, the presence of atrial fibrillation as well as treatment that the patient had received both before and after the sentinel CRAO event. A haemodynamically significant carotid artery stenosis was defined as a carotid artery stenosis of more than 70% according to either ultrasound or angiographic criteria.8 Information on revascularisation procedures was also recorded. In addition, passive ascertainment was used to determine patient outcomes in the years following the initial event with particular emphasis on subsequent vascular events of either the retinal arteries or other vascular beds.
For the purpose of analysis, descriptive statistics were used. Continuous variables are expressed as mean±SD whereas dichotomous variables are expressed as a proportion or percentage.
Results
Thirty-three patients, mean age 73±9.7 years, with acute loss of vision due to CRAO were reviewed.
Vascular risk factors and co-morbidities
Hypertension was the most common vascular risk factor in subjects with CRAO (Table 1) followed by hyperlipidemia. The diagnosis of these was based on documented past medical history recorded in case notes. On average, most patients had 2.4±1.6 number of known vascular risk factors at the time of CRAO diagnosis. Table 1 summarises the vascular risk factors present in our study population.
At the time of CRAO diagnosis, five patients (15%) had already had an episode of amaurosis fugax and one had a branch retinal artery occlusion in the same eye (3%) before the CRAO event. Five patients (15%) had a history of stroke or transient ischaemic attack on the ipsilateral side and 12 (36%) had ischaemic heart disease.
Previously undiagnosed vascular risk factors and treatment
Twenty-one patients (64%) had at least one new vascular risk factor found after the retinal occlusive event. Hyperlipidemia was the most common undiagnosed vascular risk factor at the time of the sentinel CRAO event found in 12 patients (36%), followed by hypertension nine patients (27%), and diabetes mellitus in four patients (12%) (Table 2). Eighteen patients (55%) required either a new medication or upward titration of their dose.
In two instances, patients presenting with CRAO had more complex risk factors. The youngest patient, aged 50 years at presentation, had a history of hypertension, hyperlipidemia, and on further investigation was found to have elevated homocystein levels. One patient had uncontrolled accelerated hypertension from underlying renal artery stenosis that was diagnosed after the CRAO event and referral to an internal physician.
Carotid artery disease and cardiac diseases
Duplex doppler examination of the carotid arteries was carried out on all patients. Five patients (15%) had significant carotid artery stenosis (⩾70%) ipsilateral to the retinal occlusion; four proceeded with carotid endarterectomy, and one patient, because of perioperative risks, had carotid stenting (Table 3). Four patients (12%) had moderate carotid narrowing (51–69%); one of these individuals proceeded with carotid endarterectomy after consultation with a vascular surgeon and a stroke physician, given recurrent ipsilateral ischaemic symptoms. All the carotid recanalisation surgery was performed within 1 month of the sentinel CRAO event. One patient had an ipsilateral CEA before presentation with CRAO.
A transthoracic echocardiogram was performed in 29 of 33 patients (88%) to investigate for possible cardio-embolic causes of retinal occlusion.9 In one case, echocardiogram revealed previously undiagnosed moderate left atrial enlargement associated with atrial fibrillation, resulting in oral anticoagulation.
Systemic vascular events in follow-up period
The average follow-up period in this study is 35.4±34. 9 months (range 4–132). One patient (3%) experienced a stroke on the contralateral vascular territory 5 years later. One patient (3%) developed chest pain and shortness of breath 8 months after the CRAO presentation. Subsequent investigation showed she had an anterior myocardial infarction and she was treated with angioplasty.
Discussion
Like stroke and ischaemic heart disease, retinal ischaemia due to CRAO is caused by a platelet fibrin clot or in rare instances, pure cholesterol emboli.10 The ultimate source of these thrombi or emboli is aetherosclerotic disease and thus the same risk factors that predispose to aetherosclerotic disease are prevalent in patients with CRAO.11 In our own cohort, hypertension is the most prevalent risk factor at presentation, a similar finding to a recent large case series of CRAO.12 However, a significant proportion of individuals also had carotid artery stenoses that were amenable to immediate carotid intervention, in addition to a proportion of individuals with 50–69% stenoses where carotid intervention is a potential option.13
In addition to known risk factors documented before presentation of CRAO, there are also a significant number of individuals with previously undiagnosed vascular risk factors. In some individuals escalation of existing antihypertensive medication or the addition of further vascular preventative medication was required. Although it is not known whether the use of antihypertensives, antiplatelets or cholesterol-lowering agents will reduce the risk of a subsequent CRAO based on definitive randomised controlled trials, such agents are accepted as standard clinical practise for the secondary prevention of stroke or ischaemic heart disease.14, 15 In our study, one patient developed a cerebral stroke following the CRAO and one patient developed symptoms related to ischaemic heart disease. Therefore the presentation of a CRAO while being rare given its overall incidence, nevertheless has serious complications associated with the increased subsequent incidence of vascular disease involving important end organs such as the brain and heart.
CRAO as a disease process is considered an ocular emergency, however, treatment in the past has been limited by the lack of effective acute treatments supported by robust evidence.16 This coupled with its low incidence has resulted in clinicians ignoring the potential for secondary prevention of further ocular ischaemic events as well as stroke and heart disease. This is reflected by the fact that although there is voluminous guideline level literature on the treatment of heart disease, stroke and peripheral vascular disease, equivalent literature is lacking for CRAO. Our audit of a prospective cohort of CRAO patients is important for two reasons. First, it supports the existing literature that shows the presence of a significant burden of pre-existing vascular risk factors that are present before CRAO headed by hypertension.1, 3, 9, 17 In addition, our study also shows that in a significant proportion of cases the control of such risk factors is inadequate as 55% (18 of 33 patients) required either the addition or escalation of existing macrovascular preventative medications in the follow-up period following CRAO. The second important aspect of this study is that it demonstrates that CRAO is not a benign disease, but is a marker for subsequent vascular disease, such as stroke and ischaemic heart disease with known attendant significant morbidity and mortality. The fact that 6% of CRAO patients in our cohort went on to have a cerebral stroke mirrors in magnitude the risk of transient ischaemic attacks (TIA) proceeding on to a completed stroke.4 This calls for a need for aggressive pharmacotherapy for secondary prevention of an ischaemic event. Referral to a dedicated vascular physician whether it be a stroke neurologist, cardiologist or internal medicine specialist would facilitate such therapy in addition to ongoing ophthalmology input.
Not only is CRAO followed by ischaemia in other organs but it is often preceded by warning symptoms of retinal ischaemia. In our cohort, 18% of individuals (6 of 33 patients) had either preceding symptoms of transient monocular blindness or evidence of branch retinal artery occlusion. Over half of the patients (51%) also had previous end organ ischaemia, such as ischaemic heart disease or stroke. These sentinel events if recognised early afford the clinician not only the opportunity to prevent a CRAO, but by starting antiplatelets, antihypertensive or cholesterol-lowering agents also reduce the burden of subsequent disease in other vascular beds, such as subsequent stroke or retinal artery occlusion.12, 18, 19
Conclusion
A high proportion of patients presenting with CRAO often have an undiagnosed vascular risk factor. In this study, 64% of patients have at least one undiagnosed vascular risk factor and a significant proportion required either the addition or escalation of existing macrovascular preventative medications and 18% required surgical intervention for carotid recanalisation. As this population is at high risk of secondary ischaemic events, risk factor modification is prudent to prevent further ischaemic events.
References
Rumelt S, Dorenboim Y, Rehany U . Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol 1999; 128: 733–738.
Chen C, Lee A . Management of acute central retinal artery occlusion. Nat Clin Pract Neurol 2008; 4: 376–383.
Schmidt D, Schulte-Mönting J, Schumacher M . Prognosis of central retinal artery occlusion: local intraarterial fibrinolysis versus conservative treatment. AJNR Am J Neuroradiol 2002; 23: 1301–1307.
Giles MF, Rothwell PM . Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2007; 6: 1063–1072.
Giannuzzi P, Temporelli PL, Marchioli R . Global secondary prevention strategies to limit event recurrence after myocardial infarction: results of the GOSPEL study, a multicenter, randomized controlled trial from the Italian Cardiac Rehabilitation Network. Arch Intern Med 2008; 168: 2194–2204.
Chawluk JB, Kushner MJ, Bank WJ, Silver FL, Jamieson DG, Bosley TM et al. Atherosclerotic carotid artery disease in patients with retinal ischemic syndromes. Neurology 1988; 38: 858.
Recchia FM, Brown GC . Systemic disorders associated with retinal vascular occlusion. Curr Opin Ophthalmol 2000; 11: 462–467.
North American Symptomatic Carotid Endarterectomy Trial (NASCET) Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325: 445–453.
Gaunt M, Davis P, Lee AG, Lee MS . Getting to the heart of the matter. Surv Ophthalmol 2008; 53: 636–640.
Babikian V, Wijman C, Koleini B . Retinal ischemia and embolism. Etiologies and outcomes based on a prospective study. Cerebrovasc Dis 2001; 12: 108–113.
Wong TY, Klein R . Retinal arteriolar emboli: epidemiology and stroke risk. Curr Opin Ophthalmol 2002; 13: 142–146.
Schmidt D, Hetzel A, Geibel-Zehender A, Schulte-Mönting J . Systemic diseases in non-inflammatory branch and central retinal artery occlusion-an overview of 416 patients. Eur J Med Res 2007; 12: 595–603.
Barnett H, Taylor D, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339: 1415–1425.
Graham GD . Secondary stroke prevention: from guidelines to clinical practice. J Natl Med Assoc 2008; 100: 1125–1137.
Paciaroni M, Hennerici M, Agnelli G, Bogousslavsky J . Statins and stroke prevention. Cerebrovasc Dis 2007; 24: 170–182.
Fraser SG, Adams W . Interventions for acute non-arteritic central retinal artery occlusion. Cochrane Database Syst Rev 2009; (1): CD001989.
Hayreh S, Zimmermanb B, Kimuraa A, Sanon A . Central retinal artery occlusion. Retinal survival time. Exp Eye Res 2004; 78: 723–736.
Romano JG, Sacco RL . Progress in secondary stroke prevention. Ann Neurol 2008; 63: 418–427.
Campbell DJ . A review of Perindopril in the reduction of cardiovascular events. Vasc Health Risk Manag 2006; 2: 117–124.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rudkin, A., Lee, A. & Chen, C. Vascular risk factors for central retinal artery occlusion. Eye 24, 678–681 (2010). https://doi.org/10.1038/eye.2009.142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.142
Keywords
This article is cited by
-
Statin Treatment on Cardiovascular Risk After Retinal Artery Occlusion: A Historical Cohort Study
Journal of Epidemiology and Global Health (2023)
-
Retinale arterielle Verschlüsse (RAV)
Die Ophthalmologie (2023)
-
Internal limiting membrane detachment in acute central retinal artery occlusion: a novel prognostic sign seen on OCT
International Journal of Retina and Vitreous (2021)
-
Development of visual acuity under hyperbaric oxygen treatment (HBO) in non arteritic retinal branch artery occlusion
Graefe's Archive for Clinical and Experimental Ophthalmology (2020)
-
Leitlinie von DOG, RG und BVA: Retinale arterielle Verschlüsse (RAV)
Der Ophthalmologe (2017)