Abstract
Trachoma has been known since pre-Pharaonic times and this infectious cause of blindness is targeted by the World Health Organization (WHO) for elimination by 2020. During the 19th century, trachoma was a major political problem in European countries as an important cause of disability in both soldiers and among the urban poor in the slums created by the Industrial Revolution. Even 100 years ago, trachoma was a major problem worldwide. Trachoma has now been eliminated from most developed regions, but the disease still affects some 150 million people in 56 developing countries. This paper reviews our current understanding of trachoma and the key strategies for the elimination of trachoma as a major blinding disease.
Similar content being viewed by others
The early history of trachoma
The history and the evolution of our understanding of trachoma is important as it contains many clues that are relevant to today's efforts to finally eliminate this ancient blinding disease.
The family of Chlamydiaceae evolved with the dinosaurs and Chlamydia trachomatis first appeared with the earliest mammals about 100 millions of years ago1 (Figure 1). The ocular strains of C. trachomatis diverged from the genital strains some two–five million years ago, about the same time as Homo habilis and Homo erectus evolved. Chlamydial ocular infection has been an integral part of human evolution from its very start. With this long evolutionary history, humans and Chlamydia have clearly ‘learnt’ how to live together in relative ‘harmony’. If blinding trachoma occurred frequently throughout this period, this balance would not have been established.
The evolutionary history of Chlamydiales (Stephens;1 courtesy, Richard Stephens and the International Chlamydia Symposium).9
Modern man, Homo sapiens, evolved about 120 000 years ago, but did not start to aggregate into large communities until the end of the last ice age about 10 000 BC. As the ice-retreated people started to farm and to form the earliest settlements, the increased crowding and poor personal and community hygiene in these first settlements would have been key determinants for the development of what we recognise as trachoma—the endemic blinding disease caused by repeated episodes of ocular infection with C. trachomatis (serovars A, B, Ba, and C). Blinding trachoma needs to be differentiated from occasional episodes of self-limited chlamydial inclusion conjunctivitis (usually attributable to serovars D–K) that still continue to occur around the world, even in the most developed countries. Some 5–20% of sexually active young adults in western countries have genital chlamydial infection.2
Trachoma was first seen and probably well known in the early settlements in the four great river valleys that sheltered the development of early civilisations; those of the Yangtze and Hwang Ho (Yellow) in China, the Indus in South Asia, and the Euphrates and Tigris in Mesopotamia—the so-called ‘fertile crescent’ along the Nile in Egypt.
References to trachoma in China date back to 2700 BC when Emperor Huang Ti Nei Ching had surgery for trichiasis.3 Trachoma existed in Sumeria in the Bronze Age and epilation forceps, dating from around 2000 BC, were found in Ur.4 The Indian surgeon Susruta (between 1000 and 500 BC), describes changes in the eyelids and trichiasis surgery.5
Trachoma was common and well recognised in ancient Egypt, and the treatment of eye diseases including trachoma feature prominently in the Ebers papyrus (1550 BC).5 Topical treatments included a range of biologic and mineral preparations. Many of the recommended Pharonic remedies are still in traditional use in Egypt.6
Ophthalmia was well known to the Ancient Greeks and described by Hippocrates (460–380 BC).5 It became particularly troublesome during the long siege of Athens during the Peloponnesian War (431–414 BC) when Athenians were crowded within their city walls.7 Plato (427–347 BC) suggested that ophthalmia was contagious and Aristotle (384–322 BC) concluded that one could catch trachoma just by looking at someone who was infected. Celsus (25 BC to 50 AD), better known for the four cardinal signs of inflammation, also described trachoma and the subsequent development of trichiasis.8 Dioscorides (40–90 AD) was the first to use the Greek word ‘trachoma’ to describe the roughness of everted eyelids. Galen (129–200 AD), a physician who also couched for cataracts, introduced the term ‘trichiasis’ and the four stages of trachoma: psorophthalmia, choma (trachoma), sycosis, and tylosis (roughly translated as itch, rough, scarred, and trichiasis).5 These foreshadowed the classification MacCallan of nearly 2000 years later.9
Arab authors called trachoma ‘jarab’ (or scabies) and built on the translated texts of the Greek and Roman authors.10 Honian ibn Is’hag (808–873 AD), a Christian physician in Baghdad, was the first to describe pannus or ‘sabal’. The early European texts included one by Peter the Spaniard (1210–76), a Portuguese physician who became Pope John XXI, and others from Italy, France, Germany, Holland, Spain, Portugal, and England.7, 10 They reiterated that the teachings of Galen preserved through the Arabic texts, although it can be hard to differentiate blinding trachoma from untreated bacterial or viral conjunctivitis.
However, trachoma reached much greater prominence in 19th century Europe, with the crowding of the rural poor in urban slums as they moved to the cities and towns during the Industrial Revolution, and especially among the unprecedentedly large armies that fought across Europe and were often quartered in appalling conditions.7
Trachoma really came to prominence in Europe during the Egyptian Campaign (1798–1802) when devastating epidemics of ophthalmia spread rapidly through the French and British troops.11 The French physicians and military surgeons did not consider ophthalmia to be contagious, although the English believed that it was. The ophthalmia was often complicated by severe bacterial infection that led to corneal ulceration and perforation.
Outbreaks of the Egyptian ophthalmia occurred as troops returned to Europe and then it spread rapidly to the civilian population.12 It continued to rage through the armies of Europe as they fought back and forth through the Napoleonic Wars and the other conflicts throughout the 19th century.7 Eventually, stringent military hygiene measures curbed the spread on infection so that by the First World War (1914–1918), trachoma was no longer a military problem, although it was still common in civilians in Egypt and in many parts of Europe.11
The response to trachoma
The response to trachoma led to the development of the separate speciality of ophthalmology, many institutions, and international endeavours. In 18th century Britain, eye surgery had the worst reputation for quackery and was provided by travelling surgeons, but in the early 1800's, ophthalmology became the first, and some would say the most successful, medical specialty.13 The explosive impact of the Egyptian ophthalmia elevated the importance of eye disease, so that every medical practitioner needed to know something about it.12 The army formed separate eye hospitals for soldiers with ophthalmia. This was replicated in civilian life; the London Dispensary for Curing Diseases of the Eye and Ear was founded in 1805 and ultimately became Moorfields Eye Hospital.14 In Britain, 52 eye or eye and ear hospitals were established to deal with trachoma,15 and eye hospitals rapidly appeared throughout Europe, the new world, and even in Australia.16 Institutions were established for those blinded by ophthalmia, and in the 1870s, special trachoma schools were established for children with trachoma, first in London and then elsewhere.7 Doctors trained in the management of eye disease were needed to staff the new eye hospitals, and suddenly ophthalmology was created and became a mainstream medical activity.17 After the invention of the ophthalmoscope by von Helmholtz (1821–1894) in 1850, physicians started to examine the eye in even greater detail, and the ophthalmoscope became as much a tool of medicine as the stethoscope or the tongue depressor. The International Congress of Ophthalmology in Brussels in 1857 was one of the first international medical meetings ever held.18 It was convened to discuss trachoma and the new ophthalmoscope.
Trachoma gradually disappeared from the developed countries during the 20th century. Some countries, most notably the United States, instituted tight quarantine and exclusion procedures against immigrants who had trachoma.7, 19, 20 Most of the disappearance of trachoma was due to improved living standards, although trachoma control activities including the use of newly available sulpha drugs were also important and were usually continued until the very last cases disappeared.21 It was not a question of aiming to have a prevalence of active trachoma and all below a certain level in these countries; it was not acceptable to have any active trachoma period. Trachoma did not finally disappear from Western Europe and North America until the 1950s.22 An exception was the southwest United States where trachoma continued to be a problem in the Indian populations until improving socioenvironmental conditions, and the use of sulpha drugs led to its decline in the 1960s and its eventual disappearance in the 1980s.23
It is anomalous that Australia should be the only developed country listed by the WHO as having endemic blinding trachoma,24 and trachoma still flourishes in Aboriginal communities in outback Australia.25 Unfortunately, a detailed discussion of this is beyond the scope of this presentation.
A hundred and fifty years ago, Africans and Afro-Americans were noted to have much lower rates of trachoma than the Europeans among whom they lived and worked.7, 20, 26 At that time, trachoma was also believed to be rare in sub-Saharan Africa, although Africans in the West Indies had some trachoma. This is interesting given the high rates of trachoma found in sub-Saharan Africa later in the 20th century; was the initial assessment just a mistake or did trachoma rates really increase with colonisation and the formation of larger settlements in sub-Saharan Africa?
Clinical diagnosis
Trachoma has two major phases: active or inflammatory trachoma with the variable presence of demonstrable infection and cicatricial or late trachoma with tarsal scarring entropion and trichiasis. In late trachoma, inflammation is variable, and Chlamydia is infrequently seen.
In an endemic area, a well-established case of active trachoma can be very easy to diagnose. Difficulties occur with the differentiation of borderline cases from normal; or sometimes, distinguishing cases of severe inflammatory trachoma from acute bacterial or viral conjunctivitis. The detection of trichiasis only requires careful examination. However, the diagnosis of trachoma in areas of low endemicity can be much more difficult, although severe tarsal scarring, corneal pannus with Herbert's pits, and upper lid trichiasis are pathonomonic in any setting.
Hippocrates likened the lid in trachoma to a cut ripe fig (red, swollen, and with white dots) and Galen foreshadowed the MacCallan classification9 that became the standard through much of the 20th century. In 1966, a new WHO grading system was developed that used nine signs.27 This was revised some in 1981,28 and in 1987, the WHO simplified grading system with five signs was developed, particularly for use by field workers.29 Unfortunately, the simplified grading often has been used in detailed studies looking at the correlation of infection and clinical signs where a more detailed assessment of clinical diseases would be preferred.
A recent WHO recommendation is for field studies to collect data only for three signs, TF, TT, and CO: TF (trachomatous inflammation follicular) is used to assess the need for trachoma control programs and monitor results; TT (trachomatous trichiasis) indicates those who need surgery; and CO (corneal opacity) is used as an indicator of the burden of blindness and visual impairment due to trachoma.30 Around 2400 years after Hippocrates, our thinking has evolved from trachoma being a relatively simple disease with a roughened lid and later trichiasis, through increasing degrees of complexity with a multiplicity of terms only to return to the basic definition of a disease with a roughened lid (TF) and trichiasis (TT).
Laboratory diagnosis
Many studies in the early 1800s had shown that ophthalmia (or trachoma) could be transferred by ocular discharge from one eye to another.31 Although this convinced many of the contagious nature of trachoma, it was not until the identification of bacteria in the 1860s, the notion that trachoma could be acquired from the myasm was finally dispelled. Halberstaedter and von Prowazek32 in 1907 confirmed the infective nature of trachoma when they identified the Giemsa-stained intracytoplasmic inclusions. Although Giemsa cytology was essentially 100 per cent specific, it had a low sensitivity, and organisms could not be shown in every case of clinical trachoma; as we will see, this has continued to be a problem with even the most sensitive tests and relates to the immunopathogenesis of trachoma.
The trachoma ‘virus’ was first cultured in a chick embryo in 1957.33 Cultured Chlamydia provided antigen for serologic tests, enabled the serotyping of isolates, and stimulated the search for a vaccine.4 Chlamydia culture in fertilised eggs was difficult and expensive and tissue culture methods were developed first using a monolayer of McCoy cells.34
The development of monoclonal antibody technology led to direct fluorescent antibody (DFA) cytology that used fluoroscein-conjugated monoclonal antibodies to detect free chlamydial elementary bodies.35 Enzyme immunoassays (EIA) were also developed for direct antigen detection and a number of commercial EIA kits became available.36 An advantage of a cytology specimen over a swab for culture or other tests was that the adequacy of each cytology specimen could be assessed. About 10 per cent of specimens might be inadequate.37 Specimens taken 5 min apart had a discordant rate of about 10 and 25% for those taken 2 days.38 This inconsistency in positive specimens was attributed to both sampling and biological variation in shedding.
Polymerase chain reaction (PCR) using nucleic acid amplification further improved the detection of Chlamydia, and minute amounts of chlamydial DNA could be detected.39 This gave a significant increase in sensitivity, but also increased test complexity and cost. Some PCR tests use RNA instead of DNA.40 The increased sensitivity of PCR has led to some specific problems in the study of trachoma. In a clinic, one patient is seen at a time and usually examiners will glove and specimens are collected using a semisterile technique. However, trachoma fieldwork was conducted under very different circumstances often with little regard for sterile technique. This was not a problem with the earlier relatively insensitive tests, but even a tiny bit of carried over DNA can give a false-positive PCR test. This was clearly an issue with some early reports of PCR in trachoma, although most studies now use appropriate precautions against contamination.4
The developments of quantitative PCR allowed estimates of the number of organisms present.41 Initial reports were confusing as many people had low levels of detectable organism irrespective of their clinical status, probably due to specimen contamination. Nevertheless, there is a clear picture that both young children and those with more severe disease have the highest levels of infection.
Authors have commented on the poor correlation between clinical examination and PCR, particularly in areas with low prevalence of trachoma.42, 43, 44, 45 As detectable chlamydial infection rapidly declines after mass antibiotic treatment, some have suggested that a reliance on clinical examination would lead to unnecessary antibiotic treatment in communities with clinically active trachoma but with no or low levels of infection.42 It is true that a large proportion of people with active trachoma (TF), including some of those with intense inflammation (TI), do not have demonstrable organism on spot testing. However, this reflects the pathogenesis of trachoma as an immune-mediated disease, rather than some new divergence from the ‘germ theory’.
Trachoma is an immune-mediated disease
The immunopathology of trachoma is a chronic, delayed-type hypersensitivity reaction maintained by the intermittent, but repeated, presence of chlamydial antigen; trachoma is like ‘chronic poison ivy’.
Repeated episodes of reinfection are of fundamental importance in maintaining the presence of chlamydial infection, and thus the presence of chlamydial antigens.4 In developed countries, blindness from chlamydial conjunctivitis (trachoma) is no longer seen, even though trachoma was a major problem in that population within the last century and Chlamydia infections are still prevalent.2 Why do people become blind from trachoma in areas where reinfection occurs often, but do not become blind in areas where chlamydial reinfection is rare? The genetics of the population do not change; the key is the occurrence of episodes of reinfection.
The chlamydial infection in trachoma occurs in the conjunctival epithelium; but the disease in trachoma occurs in the subepithelial tissues.4 Chlamydial infection initiates a mild, acute inflammatory response initially indistinguishable from other causes of infectious conjunctivitis. However, within days, lymphocytes accumulate and form the characteristic lymphoid follicles. A dense mantle of lymphocytes and inflammatory infiltrate forms between the follicles. With time, this infiltrate is replaced with fibrous tissue and scarring.
A single inoculum of C. trachomatis leads to an acute, self-limited episode of ‘inclusion conjunctivitis’ both in humans and in monkeys.46, 47, 48, 49, 50, 51 Chlamydia can be cultured within 1 week of inoculation, and acute follicular conjunctivitis develops rapidly and resolves over a 2–4-month period. Cultures are usually negative after about 1 month.
After recovery from primary infection there is some partial protection or immunity to rechallenge infection. Repeated inoculation experiments are not often carried out in humans.52 However, in the monkey model, repeated reinoculation has been shown to produce a marked, persistent follicular disease that is sustained for as long as the reinoculation is continued and ultimately leads to tarsal scarring.51 Even though repeated weekly reinoculation with live organism is needed to maintain chronic disease, after the first few months infection cannot be shown.
The chlamydial genome codes for 894 proteins.53 However, only a small number of these proteins elicit an immune response. The major antibody response is directed to the major outer membrane protein (MOMP).54 The chlamydial Hsp60 antigen generates a weaker antibody response, but unlike other chlamydial antigens, it stimulates a marked inflammatory reaction when instilled into the eyes of sensitised animals.55, 56 The response is a cell-mediated or delayed-type hypersensitivity reaction. These clinical and immune responses are indistinguishable from those seen with chlamydial infection itself.57, 58
The concept of trachoma as a disease of delayed-type hypersensitivity totally changes one's thinking about the interaction between infection and disease.4 The classic examples of delayed-type hypersensitivity are poison ivy or other contact allergies. The mere touch of a leaf by somebody previously sensitised to poison ivy will lead to the development of a painful inflammatory skin reaction within a few hours that lasts for several weeks. The antigenic contact is fleeting and occurs days or weeks before the inflammatory reaction finally resolves, and so there can be marked skin changes in the absence of any long gone antigen. To translate this concept to trachoma, one would need only the occasional, transient infection of a few epithelial cells with Chlamydia to allow for the elaboration and release of sufficient Hsp60 antigen to maintain active inflammation. An important corollary is that as the disease is a cell-mediated immune response, the presence or absence of a humoral response seems to be of relatively little importance.
Understanding the detailed cellular and molecular events involved in trachoma is a rapidly evolving field and the development of new technology can lead to exciting advances in knowledge.59 The identification of Chlamydia by Giemsa cytology and confirmation that trachoma resulted from infection opened the way for the first trachoma vaccine studies in 1907.46 Vaccine research received further impetus in the 1960s once Chlamydia could be cultured.26, 60 Vaccination gave partial, short-term protection that was comparable to that following recovery from a primary ocular infection. In some instances, immunised individuals developed more severe disease. A commercial vaccine was developed, but not used.61 Further work in the 1980s failed to give better protection than ocular infection despite inducing strong and specific, ocular and systemic, antibody and cell-mediated immunity responses.4 Given this experience, I do not see a viable trachoma vaccine in the foreseeable future.
The epidemiology of trachoma and reinfection
Trachoma is a disease that occurs in clusters. Some have said ‘trachoma is a lumpy disease’. These pockets or lumps may be countries (currently 56 of 194, Figure 2), regions, districts, villages or even household clusters within villages; but ultimately trachoma is a disease of individual families. Epidemiology can describe where trachoma occurs, but more importantly, why some are affected and others are not and so explain the lumpiness and the basis for family clustering.
Maps of available active trachoma data in children by WHO region (Polack et al;62 © WHO, reproduced with permission).
Although there was much confusion in the 19th century about the definitions of trachoma (its cause, course, and complications) by the 1900s, the family clustering of trachoma was well recognised.7 In 1963, Thygeson26 said ‘Since trachoma is known to spread chiefly in the home, the characteristics of the home environment are important … One can almost pick out on sight the homes where trachoma will be found rampant’. There are copious reports from around the world confirming family clustering of trachoma. Studies of the serotype, and now the genotype of Chlamydia, give further evidence of family clustering60, 63, 64, 65, 66, 67 as does the pattern of the re-emergence of chlamydial infection after community-wide treatment.68
Active trachoma is a disease of early childhood, or as Fred Hollows succinctly put it: ‘Trachoma is a disease of the crèche’. The first infection can occur shortly after birth and the bulk of chlamydial infection occurs in children under 5 years, with infants 3–9 months of age shedding the highest number of organisms.41, 69, 70 Children in their first year of life are a major source of infection in their families, especially as many treatment programs only treat children above the age of 1 year. This leaves untreated the most potent reservoir of infection in the family, the youngest children—the family-based, infantile fountains of infection.4 The peak prevalence of infection occurs well before the peak of active trachoma that usually occurs between 2 and 5 years depending on the overall level of endemicity, and usually shows a rapid decline after the age of 7–10 years (Figure 3).
Age-specific prevalence of trachoma in Australian Aborigines71 (courtesy, Royal Australian and New Zealand College of Ophthalmologists).
One key to understanding trachoma was the recognition of the importance of repeated episodes of reinfection.4, 60 Jones72 related the duration and severity of trachoma to the level of ‘ocular promiscuity’ within a family and the interaction of the individual with the family ‘pool of infection’.
Some longitudinal studies have identified children who appear to have persistently severe infection.64, 73 It is unclear whether they form a genetically susceptible subgroup of children who cannot resolve infection or whether environmental factors that facilitate reinfection were important.4, 74 However, without infection, these children would not have disease.75
In general, girls and women are more affected by trachoma than boys and men, as generally women have a more prolonged exposure to the ‘infectious pool’ than men.4 Young girls often have a major responsibility for caring for their younger siblings and mothers continue to interact with their children, whereas boys and men leave the ‘crèche’ to tend animals or work in the fields. However, the predominance of trachoma in women is not universal, and in other areas, the rates of trachoma are similar for men and women.
Trachoma persists when hygiene is poor
There are many examples where trachoma progressively decreased over the last century as living conditions gradually improved.4 An initial lowering of the intensity of active trachoma was noted, followed by a reduction in the levels of infection, then the incidence, a gradual decrease in prevalence, and ultimately a decrease in the cicatricial complications.60, 72 This sequence is important as the prevalence of active trachoma (TF) is the indicator usually used to assess the impact of trachoma control, although this may be almost the last measure to decline.
Trachoma would ultimately disappear if one waited for general socioeconomic development to occur in every household in every village around the world, but with good conscience, we cannot sit and wait for this to happen sometime in the next 100 years. The challenge for us is to identify and address the specific conditions that expose an individual to an increased risk of trachoma.
Experts have listed encyclopaedic catalogues of risk factors for trachoma that included ‘poverty, dirt, crowding, and ignorance’.76 Although these lists cover almost every conceivable aspect of life, they do not materially advance our understanding, or foster the development of specific intervention strategies.
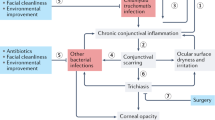
Various studies had identified more specific risk factors for trachoma. Some have looked at only one or two risk factors and have not assessed the impact of other important and potentially confounding factors.4 The most important studies are summarised and they show that the final common pathway for trachoma is a dirty face (Table 1 and Figure 4).
The interaction of trachoma risk factors. Bacterial infection hasten the progression of TF/TI to TS and TT to CO. Genetic factors may influence the progression of TF/TI to TS (Taylor,4 reproduced with permission).
The recognition of the importance of facial cleanliness provides a clear focus and target for intervention strategies and means that one does not have to rely on non-specific improvements to occur in personal and community hygiene or socioeconomic development. In any given community, one can focus on facial cleanliness and the immediate environmental barriers that impede this.
Trachoma control and the SAFE strategy
The Ancient Egyptians favoured topical treatment for trachoma and used a variety of animal, vegetable, and mineral products applied to the eye with a feather of a vulture.4 On the other hand, the Ancient Greeks related most disease to humoral causes, and so topical medications were not favoured, and treatments were used to divert inflammation from the affected eye to other organs.5 Hippocrates advocated curing ocular disease by drinking wine, bathing, purging, blood letting, and using cleansing medications. Surgery for trachoma included scarification and chemical cautery, and various surgical methods to correct entropion and trichiasis. Later, Dioscorides recommended a wide range of animal, vegetable, and mineral products to treat trachoma including copper sulphate fashioned in pencils and used a fig leaf to scarify the lid. Other treatments ranged from egg white to the ear of a mouse, mother's milk to frogs’ blood.5
From the Greco–Roman management, we inherited three pillars: surgery, general medicines, and attention to lifestyle and diet that formed the basis of the treatment of trachoma until the introduction of the first effective antimicrobials—the sulphonamides—in 1937.90 These components bear a striking similarity to the elements of the current SAFE strategy, although now our approach to trachoma control is based on the notion of repeated episodes of reinfection and the need to use multiple interventions to break the vicious cycle of reinfection (Figure 5).
Now our approach to trachoma control is based on the notion of repeated episodes of reinfection and the need to use multiple interventions to break the vicious cycle of trachoma (redrawn from West,91 with kind permission of Springer Science and Business Media).
MacCallan developed a successful trachoma control model in Egypt that was taken as the prototype programme.92 It had a central national institute to co-ordinate the activities of static and mobile eye hospitals. It used teams of nurses and doctors to conduct health education programs in schools and in the media. Once antimicrobial treatment became available, it was usually combined with these broader interventions.
The recognition of the importance of facial cleanliness in the 1980s led to calls for more targeted trachoma intervention programs that focused on the more specific health education messages. Such programs required appropriate engagement with the community,93 as the widespread use of antimicrobial agents on their own would not eliminate endemic disease.94
WHO declared the Global Elimination of Trachoma as a Blinding Disease by 2020 (GET 2020) as an achievable goal and took the lead in efforts to address trachoma.24 These included the new SAFE strategy, which stands for surgery for trichiasis (S); antibiotic treatment for active trachoma (A; tetracycline initially and now changed to azithromycin); facial cleanliness (F); and environmental improvements (E) that focused on water provision, toilets, and general cleanliness including fly reduction.95 Although SAFE is a clever acronym, it gives the elements in the reverse order to their public health importance (Figure 6).
The SAFE strategy (Francis and Turner95 © WHO, reproduced with permission).
The S component, trichiasis surgery, is built around the bilamellar tarsal rotation.96 Initial activities provided surgical kits and encouraged surgery at the community level, but more recently, the issue of quality control has surfaced with close attention to surgical technique.97, 98, 99 The recurrence rate can be reduced with the use of azithromycin at the time of surgery.100, 101
Many patients still decline trichiasis surgery, although having surgery performed in the village can increase uptake.102, 103 Other barriers included cost, the provision of care for children left at home and having an accompanying person.104, 105 Trichiasis affects the individual's physical, psychological, and environment functioning, and after surgery, each parameter shows a significant improvement.106
In 2003, the WHO24 estimated that there were 7.6 million people with trichiasis who required surgery. Even the largest current national programmes are only making a small impact on the backlog of trichiasis. Clearly, there is a need to boost capacity and expand services.99 Surgery will still be needed long after active trachoma disappears from an area as the last cohorts to have had severe active trachoma as children progressively age.
The A component of the SAFE strategy uses antibiotics to reduce the level of chlamydial infection and break the cycle of reinfection. Antibiotics will also reduce any concomitant bacterial infection. A single course of antibiotics may not totally eliminate Chlamydia in the eye or the genital tract.107, 108 If the only intervention in an endemic area were antibiotic treatment, then nothing would prevent the recrudescence of trachoma once antibiotic distribution was stopped. Thus, for sustainable trachoma control, antibiotic treatment needs to be supported by the other components of the SAFE strategy.
Azithromycin is the drug of choice and was added to the WHO list of essential drugs in 2004. It is a macrolide antibiotic derived from erythromycin. It has a broad spectrum of activity and becomes concentrated in macrophages, polymorphs, and epithelial cells.109 A single dose of azithromycin (1 g or 20 mg/kg) is as effective as a full 7-day course of doxycycline,110 or a 6-week course of topical tetracycline.111 Owing to its broad spectrum, it will treat existing respiratory, skin, and genital infections.112, 113 The community often perceives this as the major benefit of mass treatment. Azithromycin can be used during pregnancy114 and in children over 6 months, although its safety has not been tested specifically in children under 6 months.
Used as a safe, single oral dose, azithromycin is an ideal drug for trachoma. Individual diagnosis is not required and its community-based distribution can be integrated into primary health care. However, repeated treatment will be required and azithromycin treatment needs to be accompanied by the other components of the SAFE strategy. Because trachoma is ‘a disease of the crèche’, the family unit should be the unit of treatment, rather than individuals. Equally, treatment studies could focus on family treatment rather than the need to randomise whole communities.
Most studies reported so far have assessed azithromycin treatment in isolation without addressing the other components of the SAFE strategy, and many have not adequately treated the youngest children. Without the F and E components and with incomplete antibiotic coverage, trachoma could be expected to reappear quite quickly after antibiotic treatment. Reports of the efficacy of antibiotic treatment interventions vary,73, 115 and this probably reflects multiple factors including the level of endemicity, the frequency of retreatment, and the degree of coverage.
The F component, facial cleanliness, is the critical, final common pathway through which a variety of environmental factors affect the risk of trachoma, although facial cleanliness often receives the least attention.4 In part, this is because it requires a behavioural change and often this is not easy to induce. It is more straightforward for programmes to bore a well, give people a pill, or build a pit latrine. Behavioural change may need prolonged local involvement, but in the end, this is what is needed.
The landmark trial on facial cleanliness in central Tanzania increased clean faces by more than 60%, and reduced intense trachoma (TI) by 38% and active trachoma (TF) by 19%.116 TF was reduced by 42% in those children with clean faces. The effect of facial cleanliness was additional to the already significant reduction following antibiotic treatment. Recent studies further confirm the additional benefit of health education programmes promoting facial cleanliness.117, 118
The E component, environmental improvement, covers a very broad category of potential activities, and in some ways is the most difficult component of the SAFE strategy to comprehend and define.
Several studies on fly control in the Gambia showed a reduction in trachoma which was also reduced after the installation of latrines.119 However, a large study in a hyperendemic area in Tanzania showed no effect of fly control when added to azithromycin distribution.120 To my mind, the key thing about the E component is to address the barriers to keeping every child's face clean, especially crowding and children sleeping together, and of course the availability of water.
A 3-year evaluation of the SAFE strategy in southern Sudan found a dramatic reduction in active trachoma in areas with a good uptake of antibiotic, facial cleanliness, and access to water.118 Although the results lack the precision of a formal clinical trial, they reflect a real world situation. Similar success is reported from southern Zambia.121 Studies of the SAFE strategy in central Australia suggest that a sustained effort is required, and despite the extensive and expensive efforts to improve E, the environmental improvements made no additional discernable difference to the rate of active trachoma over and above A and F.122, 123
WHO and the Global Alliance review annual reports from countries implementing trachoma control activities.99 In April 2008, 38 countries reported on the progress they had made. Particularly impressive achievements have been made in Morocco, Iran, and Oman where active trachoma appears to have been eliminated. It is projected that trachoma will be eliminated as a public health problem from The Gambia in 2009, and China, Eritrea, Ghana, Myanmar, Nepal, and Vietnam by 2010.
Interesting insights come from the relative cost of implementing different components of the SAFE strategy; in one example, the S component cost 2.4% of the budget, the A component 3.4%, the F component 3.2%, but the E component took 91% of the budget.124 The huge cost for E activities reflects the enormous infrastructure costs in the provision of water and sewerage—10 times more than the others combined! This is similar to the disproportionate cost of E in the Australian study quoted above.123 A more precise focus on facial cleanliness rather than the universal provision of new wells and latrines will substantially reduce the resources required for the E component. The provision of these basic water and sanitation services is an essential component of rural development and is the goal 7 of the Millennium Development Goals.125 Many government and non-government organisations are addressing this goal. Trachoma control programmes need to encourage development agencies to prioritise trachoma areas for these activities.
Health economic data show that countries cannot afford not to address trachoma. Productivity losses due to blindness and low vision from trachoma were estimated to be US$5.3 billion in 2003 dollars.126 The disability due to trichiasis adds a further US$8 billion,127 and there are additional indirect costs and the loss of well-being (or ‘burden of disease’) due to trachoma. Furthermore, trachoma interventions are cost-effective. Trichiasis surgery costs I$13 per disability adjusted life year (DALY).127 If 80 per cent of those with trichiasis had surgery, 11 million DALY per year would be saved.128 The mass distribution of azithromycin is more cost effective than targeted household treatment,129 although this varies with the costs of the drug and its distribution. Using the donated drug, treatment is very cost-effective at $3.92 per DALY. The mass treatment of 80 per cent of the children with active trachoma would save some 4 million DALYs each year.128
Where to from now?
The SAFE strategy concisely brings together the behavioural, medical, and surgical elements required to address trachoma. The specific focus on facial cleanliness and environmental barriers coupled with a very potent antibiotic gives a highly targeted intervention to reduce both the level of infection and pressure of reinfection. This coherent strategy is based on solid evidence from field research and controlled trials. WHO and its partners have developed a comprehensive and accessible suite of background teaching and material,130 and Pfizer's Zithromax Donation Programme provides millions of doses of azithromycin each year at no cost.131
Now, we seem to have the tools, the resources, and the expression of political will needed to actually eliminate blinding trachoma. One could be tempted to say that:
‘We may therefore venture to conclude on a note of reasonable optimism and at the same time express a hope for the future. In many parts of the world, the most widespread world scourge has been or is reported to be overcome. Efficient methods of therapy are already in use and other still more effective ones may be expected in the near future. If the national campaigns already under way are continued and co-ordinated and meet with vigorous international support, our century may perhaps witness the disappearance of trachoma’ (Sidky and Freyce132).
One must reflect on this ultimately misplaced past optimism and wonder how much further advanced we really are. Nevertheless, with commitment and hard work, I am convinced that we should be able to achieve the goal of GET 2020 and make trachoma history.
References
Stephens RS . Chlamydial evolution: a billion years and counting. In: Schachter J, Christiansen G, Clarke IN, Hammerschlag MR, Kaltenboeck B, Kuo C-C et al. (eds). Chlamydial Infections; 2002. Proceedings of the Tenth International Symposium on Human Chlamydial Infections; 2002. Antalya-Turkey: International Chlamydia Symposium: San Francisco, 2002, pp 3–12.
Marrazzo JM . Epidemiology of Chlamydia trachomatis genital infections: what's new since 2002. In: Chernesky M, Caldwell H, Christiansen G, Clarke IN, Kaltenboeck B, Knirsch C et al. (eds). Proceedings of the Eleventh International Symposium on Human Chlamydial Infections; 2006. Niagara-on-the-Lake, Ontario, Canada: International Chlamydia Symposium: San Francisco, 2006, pp 59–68.
Duke-Elder S, Wybar KC . System of Ophthalmology. The Anatomy of the Visual System. The CV Mosby Company: St Louis, 1961.
Taylor HR . Trachoma: A Blinding Scourge from the Bronze Age to the Twenty-first Century. Centre for Eye Research Australia: Melbourne, 2008.
Hirschberg J . The History of Ophthalmology, in Eleven Volumes 1: Antiquity. JP Wayenborgh Verlag: Bonn, 1982.
Millar MI, Lane SD . Ethno-ophthalmology in the Egyptian delta: an historical systems approach to ethnomedicine in the Middle East. Soc Sci Med 1988; 26: 651–657.
Boldt J . Trachoma. Hodder and Stoughton: London, 1904.
Celsus AC . De Medicina (On Medicine) Celsus On Medicine Book VI. Loeb Classical Library 1935, 1935. available at: http://penelope.uchicago.edu/Thayer/E/Roman/Texts/Celsus/6*.html, accessed July 2006.
MacCallan AF . Trachoma and its Complications in Egypt. Cambridge University Press: Cambridge, 1913.
Hirschberg J . The History of Ophthalmology, in Eleven Volumes 2: The Middle Ages; The Sixteenth and Seventeenth Centuries. JP Wayenborgh Verlag: Bonn, 1985.
Meyerhof M . A short history of ophthalmia during the Egyptian campaigns of 1798–1807. Br J Ophthalmol 1932; 16: 129–152.
Vetch J . An Account of the Ophthalmia which Appeared in England since the Return of the British Army from Egypt. Longman, Hurst, Rees & Orme: London, 1807.
Davidson L . ‘Identities Ascertained’: British Ophthalmology in the First Half of the Nineteenth Century. Soc Hist Med. 1996; 9: 313–333.
Treacher Collins E . The History & Traditions of the Moorfields Eye Hospital. One Hundred Years of Ophthalmic Discovery & Development. H.K. Lewis & Co Ltd: London, 1929.
Sorsby A, Sorsby M . A Short History of Ophthalmology. Staples Press: London, New York, 1948.
Donovan P . ‘An Ornament to the City’ The Royal Victorian Eye and Ear Hospital. The Royal Victorian Eye & Ear Hospital: Melbourne, 1992.
Wilde RW . The organs of sight and hearing. Lancet 1845; pp 431–435.
Duke-Elder S . A Century of International Ophthalmology (1857–1957). Henry Kimpton: London, 1958.
Allen SK, Semba RD . The trachoma ‘Menace’ in the United States, 1897–1960—history of ophthalmology. Surv Ophthalmol 2002; 47: 500–509.
McMullen J . Trachoma, its prevalence and control among immigrants. JAMA 1913; 61: 1110–1113.
Sorsby A . The treatment of trachoma. With special reference to local sulphonamide therapy. Br J Ophthalmol 1945; 29: 98–102.
Jones BR . Changing concepts of trachoma and its control. Trans Ophthalmol Soc UK 1980; 100: 25–29.
Friederich R . Eye disease in the Navajo Indians. Ann Ophthalmol 1982; 14: 38–40.
World Health Organization. Report of the 2nd Global Scientific Meeting on Trachoma. WHO: Geneva, Switzerland, 2003.
Tellis B, Keeffe JE, Taylor HR, National Trachoma Surveillance and Reporting Unit. Surveillance report for active trachoma, 2006. Comm Dis Bull 2007; 31: 366–374.
Thygeson P . Epidemiologic observations on trachoma in the United States. Invest Ophthalmol 1963; 2: 482–489.
World Health Organization. Fourth WHO Scientific Group on Trachoma Research, (report) WHO: Geneva, 1966.
Dawson CR, Jones BR, Tarizzo ML . Guide to Trachoma Control. World Health Organization: Geneva, 1981.
Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR . A simple system for the assessment of trachoma and its complication. Bull World Health Organ 1987; 65: 477–483.
Solomon AW, Zondervan M, Kuper H, Buchan JC, Mabey DCW, Foster A . Trachoma Control—A Guide for Programme Managers. World Health Organization: Geneva, 2006.
Benedek TG . Gonorrhea and the beginnings of clinical research ethics. Perspect Biol Med 2005; 48: 54–73.
Halberstaedter L, von Prowazek S . Uber zelleinschusse parasitarer natur beim trachom (On cell inclusions of a parasitic nature in trachoma). Arb K Gesundh Amt 1907; 26: 44–47.
T’ang FF, Chang HL, Huang YT, Wang KC . Studies on the etiology of trachoma with special reference to isolation of the virus in chick embryo. Chin Med J 1957; 75: 429–447.
Gordon FB, Quan AL . Isolation of the trachoma agent in cell culture. Proc Soc Exp Biol Med 1965; 118: 354–359.
Wilson MC, Millan-Velasco F, Tielsch JM, Taylor HR . Direct-smear fluorescent antibody cytology as a field diagnostic tool for trachoma. Arch Ophthalmol 1986; 104: 688–690.
Caldwell HD, Schachter J . Immunoassay for detecting Chlamydia trachomatis major outer membrane protein. J Clin Microbiol 1983; 18: 539–545.
Taylor HR, Rapoza PA, West S, Johnson S, Muñoz B, Katala S et al. The epidemiology of infection in trachoma. Invest Ophthalmol Vis Sci 1989; 30: 1823–1833.
Taylor HR, Siler JA, Mkocha HA, Muñoz B, Velez V, DeJong L et al. The microbiology of endemic trachoma—a longitudinal study. J Clin Microbiol 1991; 29: 1593–1595.
Dean D, Pant CR, O’Hanley P . Improved sensitivity of a modified polymerase chain reaction amplified DNA probe in comparison with serial tissue culture passage for detection of Chlamydia trachomatis in conjunctival specimens from Nepal. Diagn Microbiol Infect Dis 1989; 12: 133–137.
Yang JL, Schachter J, Moncada J, Habte D, Zerihun M, House JI et al. Comparison of an rRNA-based and DNA-based nucleic acid amplification test for the detection of Chlamydia trachomatis in trachoma. Br J Ophthalmol 2007; 91: 293–295.
Solomon AW, Holland MJ, Burton MJ, West SK, Alexander NDE, Aguirre A et al. Strategies for control of trachoma: observational study with quantitative PCR. Lancet 2003; 362: 198–204.
Solomon AW, Peeling RW, Foster A, Mabey DCW . Diagnosis and assessment of trachoma. Clin Microbiol Rev 2004; 17: 982–1011.
Miller K, Schmidt G, Alemayehu W, Yi E, Cevallos V, Donnellan C et al. How reliable is the clinical exam in detecting ocular chlamydial infection. Ophthalmic Epidemiol 2004; 11: 255–262.
Wright HR, Taylor HR . Clinical examination and laboratory tests for estimation of trachoma prevalence in remote settings. Lancet 2006; 6: 7–8.
Baral K, Osaki S, Shreshta B, Panta CR, Boulter A, Pang F et al. Reliability of clinical diagnosis in identifying infectious trachoma in a low-prevalence area of Nepal. Bull World Health Organ 1999; 77: 461–466.
Nicolle C, Cuenod A, Baizot L . Etude experimentale du trachome. Arch Instit Pasteur de Tunis 1913; 4: 157–182.
Dawson CR, Wood TR, Rose L, Hanna L, Thygeson P . Experimental inclusion conjunctivitis in man. III. Keratitis and other complications. Arch Ophthalmol 1967; 78: 341–349.
Thygeson P, Dawson C, Hanna L, Jawetz E, Okumoto M . Observations on experimental trachoma in monkeys produced by strains of viruses propagated in yolk sac. Am J Ophthalmol 1960; 50: 907–918.
Wang S-P, Grayston JT . Pannus with experimental trachoma and inclusion conjunctivitis agent infection of Taiwan monkeys. Am J Ophthalmol 1967; 63: 1133–1145.
Dawson CR, Jawetz E, Thygeson P, Hanna L . Trachoma viruses isolated in the United States. 4. Infectivity and immunogenicity for monkeys. Proc Soc Exp Biol Med 1961; 106: 898–902.
Taylor HR, Johnson SL, Prendergast RA, Schachter J, Dawson CR, Silverstein AM . An animal model of trachoma II. The importance of repeated reinfection. Invest Ophthalmol Vis Sci 1982; 23: 507–519.
Mitsui Y, Higai H, Kitamuro T . Free toxic substance of trachoma virus. Arch Ophthalmol 1962; 68: 651–653.
Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L et al. Genome sequence of an obligate intracellular pathogen of human: Chlamydia trachomatis. Science 1998; 282: 754–759.
Caldwell HD, Stewart S, Johnson S, Taylor H . Tear and serum antibody response to Chlamydia trachomatis antigens during acute chlamydial conjunctivitis in monkeys as determined by immunoblotting. Infect Immun 1987; 55: 93–98.
Taylor HR, Johnson SL, Schachter J, Caldwell HD, Prendergast RA . Pathogenesis of trachoma: the stimulus for inflammation. J Immunol 1987; 138: 3023–3027.
Taylor HR, Maclean IW, Brunham RC, Pal S, Whittum-Hudson J . Chlamydial heat–shock proteins and trachoma. Infect Immun 1990; 58: 3061–3063.
Whittum-Hudson JA, Taylor HR . Antichlamydial specificity of conjunctival lymphocytes during experimental ocular infection. Infect Immun 1989; 57: 2977–2983.
Pal S, Pu Z, Huneke RB, Taylor HR, Whittum-Hudson JA . Chlamydia-specific lymphocytes in conjunctiva during ocular infection: Limiting dilution analysis. Reg Immunol 1991; 3: 171–176.
Bavoil PM, Wyrick PB (eds). Chlamydia: Genomics and Pathogenesis. Horizon Bioscience: Norfolk, UK, 2006.
Grayston JT, Wang S . New knowledge of Chlamydiae and the diseases they cause. J Infect Dis 1975; 132: 87–105.
Bietti G, Werner GH . Trachoma Prevention and Treatment. Charles C Thomas: Springfield, Illinois, 1967.
Polack S, Brooker S, Kuper H, Mariotti S, Mabey D, Foster A . Mapping the global distribution of trachoma. Bull World Health Organ 2005; 83: 913–919.
Nichols RL, Von Fritzinger K, McComb DE . Epidemiological data derived from immunotyping of 338 trachoma strains isolated from children in Saudi Arabia. In: Nichols RL (ed.) Trachoma and Related Disorders Caused by Chlamydial Agents. Excerpta Medica: Amsterdam, 1971, pp 337–357.
Taylor HR, Siler JA, Mkocha HA, Muñoz B, West S . The natural history of endemic trachoma: a longitudinal study. Am J Trop Med Hyg 1992; 46: 552–559.
Bailey RL, Hayes L, Pickett M, Whittle HC, Ward ME, Mabey DCW . Molecular epidemiology of trachoma in a Gambian village. Br J Ophthalmol 1994; 78: 813–817.
Bobo LD, Novak N, Munoz B, Hsieh YH, Quinn TC, West S . Severe disease in children with trachoma is associated with persistent Chlamydia trachomatis infection. J Infect Dis 1997; 176: 1524–1530.
Zhang J, Lietman TM, Olinger L, Miao Y, Stephens RS . Genetic diversity of Chlamydia trachomatis and the prevalence of trachoma. Pediatr Infect Dis J 2004; 23: 2057–2060.
Broman AT, Shum K, Munoz B, Duncan DD, West SK . Spatial clustering of ocular chlamydial infection over time following treatment, among households in a village in Tanzania. Invest Ophthalmol Vis Sci 2006; 47: 99–104.
Nichols RL, Bobb AA, Haddad NA, McComb DE . Immunofluorescent studies of the microbiologic epidemiology of trachoma in Saudi Arabia. Am J Ophthalmol 1967; 63: 1372–1408.
Ministry of Public Health. Fourteenth Report of the Memorial Ophthalmic Laboratory 1939–1944. Ministry of Public Health: Giza, Cairo, 1945.
Royal Australian College of Ophthalmologists. The National Trachoma and Eye Health Program of the Royal Australian College of Ophthalmologists. Royal Australian College of Ophthalmologists: Sydney, 1980.
Jones BR . The prevention of blindness from trachoma (Bowman Lecture). Trans Ophthalmol Soc UK 1975; 95: 16–33.
West SK, Munoz B, Mkocha H, Holland MJ, Aguirre A, Solomon AW et al. Infection with Chlamydia trachomatis after mass treatment of a trachoma hyperendemic community in Tanzania: a longitudinal study. Lancet 2005; 366: 1296–1300.
West SK, Munoz B, Lynch M, Kayongoya A, Mmbaga BBO, Taylor HR . Risk factors for constant, severe trachoma in pre-school children in Kongwa, Tanzania. Am J Epidemiol 1996; 143: 73–78.
West SK, Munoz B, Mkocha H, Hsieh YH, Lynch MC . Progression of active trachoma to scarring in a cohort of Tanzanian children. Ophthalmic Epidemiol 2001; 8: 137–144.
World Health Organization. Expert Committee on Trachoma, (third report). WHO: Geneva, 1962.
Taylor HR, Millan-Velasco F, Sommer A . The ecology of trachoma: an epidemiological study of trachoma in Southern Mexico. Bull World Health Organ 1985; 63: 559–567.
Tielsch JM, West Jr KP, Katz J, Keyvan-Larijani E, Tizazu T, Schwab L et al. The epidemiology of trachoma in southern Malawi. Am J Trop Med Hyg 1988; 38: 393–399.
Taylor HR, West SK, Mmbaga BBO, Katala SJ, Turner V, Lynch M et al. Hygiene factors and increased risk of trachoma in Central Tanzania. Arch Ophthalmol 1989; 107: 1821–1825.
Courtright P, Sheppard J, Lane S, Sadek A, Schachter J, Dawson CR . Latrine ownership as a protective factor in inflammatory trachoma in Egypt. Br J Ophthalmol 1991; 75: 322–325.
Luna EJA, Medina NH, Oliveira MB, De Barros OMD, Vranjac A, Melles HHB et al. Epidemiology of trachoma in Bebedouro State of Sao Paulo, Brazil: Prevalence and risk factors. Int J Epidemiol 1992; 21: 169–177.
Katz J, West KP, Khatry SK, LeClerq SC, Pradhan EK, Thapa MD et al. Prevalence and risk factors for trachoma in Sarlahi district, Nepal. Br J Ophthalmol 1996; 80: 1037–1041.
Sahlu T, Larson C . The prevalence and environmental risk factors for moderate and severe trachoma in southern Ethiopia. J Trop Med Hyg 1992; 95: 36–41.
Schemann J-F, Sacko D, Malvy D, Momo G, Traore L, Bore O et al. Risk factors for trachoma in Mali. Int J Epidemiol 2002; 31: 194–201.
Schemann JF, Guinot C, Ilboudo L, Momo G, Ko B, Sanfo O et al. Trachoma, flies and environmental factors in Burkina Faso. Trans R Soc Trop Med Hyg 2003; 97: 63–68.
Cumberland P, Hailu G, Todd J . Active trachoma in children aged three to nine years in rural communities in Ethiopia: prevalence, indicators and risk factors. Trans Roy Soc Trop Med Hyg 2005; 99: 120–127.
Faye M, Kuper H, Dineen BP, Bailey R . Rapid assessment for prioritisation of trachoma control at community level in one district of the Kaolack region, Senegal. Trans Roy Soc Trop Med Hyg 2006; 100: 149–157.
Mesfin MM, de la Camera J, Tareke IG, Amanual G, Araya T, Kedir AM . A community-based trachoma survey: prevalence and risk factors in the Tigray region of Northern Ethiopia. Ophthalmic Epidemiol 2006; 13: 173–181.
Abdou A, Nassirou B, Kadri B, Moussa F, Munoz BE, Opong E et al. Prevalence and risk factors for trachoma and ocular Chlamydia trachomatis infection in Niger. Br J Ophthalmol 2007; 91: 13–17.
Sorsby MA . Trachoma in Great Britain. Rev Int Trach 1939; 16: 148–155.
West S, Taylor HR . Community based intervention programs for trachoma control. Int Ophthalmol 1988; 12: 19–23.
MacCallan AF . Trachoma. Butterworth & Co. (Publishers) Ltd: London, 1936.
McCauley AP, Lynch M, Pounds MB, West S . Changing water-use patterns in a water-poor area: lessons for a trachoma intervention project. Soc Sci Med 1990; 31: 1233–1238.
Mabey DCW, Downes RM, Downes B, Bailey RL, Dunn DT . The impact of medical services on trachoma in a Gambian village: antibiotics alone are not the answer. Ann Trop Paediatrics 1991; 11: 295–300.
Francis V, Turner V . Achieving Community Support for Trachoma Control. World Health Organization: Geneva, 1993. Report no.: WHO/PBL/93.36.
Reacher M, Foster A, Huber J . Trichiasis Surgery for Trachoma—the Bilamellar Tarsal Rotation Procedure. World Health Organization: Geneva, 1993. Report no.: WHO/PBL/93.29.
El Toukhy E, Lewallen S, Courtright P . Routine bilamellar tarsal rotation surgery for trachomatous trichiasis: short-term outcome and factors associated with surgical failure. Ophthal Plast Reconstr Surg 2006; 22: 109–112.
Merbs SL, West SK, West ES . Pattern of recurrence of trachomatous trichiasis after surgery. Ophthalmology 2005; 112: 705–709.
〈http://www.who.int/blindness/publications/get2020/en/index.html.〉. Report of the Eleventh Meeting of the WHO Alliance for the Global Elimination of Blinding Trachoma, Cairo, 2–4 April, 2007; accessed June 2008.
West SK, West ES, Alemayehu W, Melese M, Munoz B, Imeru A et al. Single-dose azithromycin prevents trichiasis recurrence following surgery. Arch Ophthalmol 2006; 124: 309–314.
Zhang H, Kandel RP, Atakari HK, Dean D . Impact of oral azithromycin on recurrence of trachmatous trichiasis in Nepal over 1 year. Br J Ophthalmol 2006; 90: 943–948.
Bowman RJ, Soma OS, Alexander N, Milligan P, Rowley J, Faal H et al. Should trichiasis surgery be offered in the village? A community-randomised controlled trial of village vs community health center-basd surgery. Trop Med Int Health 2000; 5: 528–533.
Mahande M, Tharaney M, Kirumbi E, Ngirawamungu E, Geneau R, Tapert L et al. Uptake of trichiasis surgical services in Tanzania through two village-based approaches. Br J Ophthalmol 2007; 91: 139–142.
West S, Nguyen MP, Mkocha H, Holdsworth G, Ngirwamungu E, Kilima P et al. Gender equity and trichaisis surgery in the Vietnam and Tanzania national trachoma control programmes. Br J Ophthalmol 2004; 88: 1368–1371.
Courtright P . Acceptance of surgery for trichiasis among rural Malawian women. East Afr Med J 1994; 71: 803–804.
Dhaliwal U, Nagpal G, Bhatia MS . Health-related quality of life in patients with trachomatous trichiasis or Entropion. Ophthalmic Epidemiol 2006; 13: 59–66.
Rees E, Tait IA, Hobson D, Karayiannis P, Lee N . Persistence of chlamydial infection after treatment for neonatal conjunctivitis. Arch Dis Child 1981; 56: 193–198.
Centers for Disease Control and Prevention, Workowski KA, Berman SM . Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006; 55: 1–94.
Hoepelman IM, Schneider MME . Azithromycin: the first of the tissue-selective azalides. Int J Antimicrob Agents 1995; 5: 145–167.
Martin DH, Mroczkowski TF, Dalu ZA, McCarty J, Jones RB, Hopkins SJ et al. A controlled trial of a single dose of azithromycin for the treatment of chlamydial urethritis and cervicitis. N Engl J Med 1992; 327: 921–925.
Mabey D, Fraser-Hurt N, Powell C . Antibiotics for Trachoma. Cochrane Database Syst Rev 2005; CD001860.
Fry AM, Jha HC, Lietman TM, Chaudhary JSP, Bhatta RC, Elliott J et al. Adverse and beneficial secondary effects of mass treatment with azithromycin to eliminate blindness due to trachoma in Nepal. Clin Infect Dis 2002; 35: 395–402.
Shelby-James TM, Leach AJ, Carapetis JR, Currie BJ, Mathews JD . Impact of single dose azithromycin on group A streptococci in the upper respiratory tract and skin of Aboriginal children. Ped Infect Dis J 2002; 21: 375–380.
Sarkar M, Woodland C, Koren G, Einarson ARN . Pregnancy outcome following gestational exposure to azithromycin. BMC Pregnancy Childbirth 2006; 6: 18.
Solomon AW, Harding-Esch E, Alexander NDE, Aguirre A, Holland MJ, Bailey RL et al. Two doses of azithromycin to eliminate trachoma in a Tanzanian community. N Eng J Med 2008; 358: 1870–1871.
West S, Muñoz B, Lynch M, Kayongoya A, Chilangwa Z, Mmbaga BBO et al. Impact of face-washing on trachoma in Kongwa Tanzania. Lancet 1995; 345: 155–158.
Edwards T, Cumberland P, Hailu G, Todd J . Impact of health education on active trachoma in hyperendemic rural communities in Ethiopia. Ophthalmology 2006; 113: 548–555.
Ngondi J, Onsarigo A, Matthews F, Reacher M, Brayne C, Baba S et al. Effect of 3 years of SAFE (surgery, antibiotics, facial cleanliness, and environmental change) strategy for trachoma control in southern Sudan: a cross sectional study. Lancet 2006; 368: 589–595.
Emerson PM, Lindsay SW, Alexander N, Bah M, Dibba S, Faal HB et al. Role of flies and provision of latrines in trachoma control: cluster-randomised controlled trial. Lancet 2004; 363: 1093–1098.
West SK, Emerson PM, Mkocha H, Mchiwa W, Munoz B, Bailey R et al. Intensive insecticide spraying for fly control after mass antibiotic treatment for trachoma in a hyperendemic setting: a randomised trial. Lancet 2006; 368: 596–600.
Astle WF, Wiafe B, Ingram AD, Mwanga M, Glassco CB . Trachoma control in southern Zambia—an international team project employing the SAFE strategy. Ophthalmic Epidemiol 2006; 13: 227–236.
Ewald DP, Hall GV, Franks CC . An evaluation of a SAFE-style trachoma control program in Central Australia. Med J Aust 2003; 178: 65–68.
Lansingh VC . Primary Health Care Approach to Trachoma Control in Aboriginal Communities in Central Australia [PhD]. University of Melbourne: Melbourne, 2005.
World Health Organization. Report of the Ninth Meeting of the WHO Alliance for the Global Elimination of Blinding Trachoma. WHO: Geneva 21–23 March 2005. Report no.: WHO/PBD/GET/05.1.
〈http://www.un.org/milleniumgoals/〉. UN Millennium Goals; 2005, accessed on September 2006.
Frick KD, Hanson CL, Jacobson GA . Global burden of trachoma and economics of the disease. Am J Trop Med Hyg 2003; 69: 1–10.
Frick KD, Melia M, Buhrmann RB, West SK . Trichiasis and disability in a trachoma-endemic area of Tanzania. Arch Ophthalmol 2001; 119: 1839–1844.
Baltussen RM, Sylla M, Frick KD, Mariotti SP . Cost-effectiveness of trachoma control in seven world regions. Ophthalmic Epidemiol 2005; 12: 91–101.
Frick KD, Lietman TM, Holm SO, Jha HC, Chaudary JSP, Bhatta RC . Cost-effectiveness of trachoma control measures: comparing targeted household treatment and mass treatment of children. Bull World Health Organ 2001; 79: 201–207.
〈http://www.who.int/en/〉. World Health Organization, accessed June 2008.
〈http://www.pfizer.com/pfizer/history/1998.jsp〉. Exploring Our History. 1951–1999. 1998—Trachoma; 2006, accessed September 2006..
Sidky MM, Freyce MS . World distribution and prevalence of trachoma in recent years. Epidem Vital Stat Rep 1949; II: 230–277.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taylor, H. Doyne Lecture: trachoma, is it history?. Eye 23, 2007–2022 (2009). https://doi.org/10.1038/eye.2008.432
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2008.432