Abstract
Purpose
To correlate vortex vein invasion with established prognostic factors for uveal melanoma.
Methods
Enucleated eyes with a confirmed histopathological diagnosis of uveal melanoma with vortex vein invasion were identified, over a 10-year period. Established uveal melanoma prognostic factors, with tumour genetics were correlated with vortex vein invasion and patient survival.
Results
Microscopic vortex vein involvement was present in 29 of 244 (11.9%) uveal melanomas. Of 29, 6 (20.7%) tumours had macroscopic evidence of vortex vein invasion. Of 29, 14 (48.3%) tumours also showed evidence of non-vortex vein, ‘direct’ scleral invasion. 23 (79.3%) of 29 melanomas involved only the choroid. The mean maximum diameter of tumours with vortex vein invasion was 15.8 mm and the mean thickness was 9.7 mm. The uveal melanoma was a discrete nodule in 27 of 29 (93.1%) cases. Histologically, 8 of 29 tumours (27.6%) were spindle cell, 19 of 29 (65.5%) were mixed cell, and 2 of 29 (6.9%) were epithelioid cell type. Of 29, 22 (75.9%) uveal melanomas with vortex vein invasion contained extracellular matrix networks and loops. Genetic abnormalities correlated with poor prognosis were seen in 25 of 29 (86.2%) tumours with vortex vein invasion. Liver metastasis was confirmed in 19 of 29 (65.5%) patients with vortex vein invasion. No patients with uveal melanomas showing vortex vein invasion suffered orbital recurrence of disease following enucleation.
Conclusions
The trends show that vortex vein invasion is associated with a choroidal location, large tumour size, spindle cell bias, presence of extracellular matrix loops/networks and genetic markers. A higher proportion of patients with vortex vein invasion progress to develop liver metastasis compared with the general uveal melanoma population.
Similar content being viewed by others
Introduction
Uveal melanoma is the commonest primary malignant intra-ocular tumour of adulthood.1 It classically metastasizes to liver through the bloodstream. A proportion of patients will develop liver metastases, on average 10 years after diagnosis and treatment of the primary neoplasm.2 Once there is clinical evidence of metastasis, survival time is poor and averages between 5 and 9 months.3, 4, 5
Transcleral spread in uveal melanoma is associated with a poorer prognosis when compared with tumours confined to the eye.6 Extra-ocular spread can occur via a number of mechanisms. Invasion of the lumen of the vortex veins by tumour is one route by which tumour can gain access to extra-ocular compartment and the bloodstream. However, the risk factors associated with vortex vein invasion have not been well characterized to date.
Several prognostic factors have been identified and studied in uveal melanomas. Clinical prognostic factors include tumour location7 and tumour size.8 Prognostic factors identified at histology include cell type,9 the presence of extracellular matrix loops and networks,10, 11 and nucleolar size.12, 13 In addition, cytogenetic prognostic factors have also been studied, with monosomy 314, 15 and amplification of 8q15 associated with poorer survival.
Vortex vein invasion in cases of uveal melanoma can be identified at histological examination of enucleated eyes. However, the question of whether vortex vein invasion is associated with other poor prognostic indicators is not well answered at present. Hence in this study we have identified all cases of uveal melanomas with evidence of histological vortex vein invasion at our regional ocular oncology centre over a 10-year period, and investigated any correlation with other recognized prognostic factors.
Materials and methods
Since 1987, all patients seen at the ocular oncology unit at the Royal Hallamshire Hospital in Sheffield have been routinely entered into a secure database. We searched the database for all patients who had a confirmed histological diagnosis of uveal melanoma at enucleation over a 10-year period, from 1996 to 2006. All samples were obtained with informed patient consent. All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. The study was conducted with ethical approval, SSREC 94/247 and STH 13738.
A retrospective descriptive study of case notes and histopathological findings of all patients with histological vortex vein invasion was conducted. Parameters for investigation included demographical data (including age, sex), laterality, location of tumour, size, growth pattern, cell type, and the presence of extracellular matrix networks or loops. We recorded how many of the cases showed macroscopic vortex vein invasion at the pathology ‘cut-up’ bench and also examined microscopically the degree of vortex vein invasion (intra-scleral and extra-scleral), along with how many cases showed microscopic evidence of non-vortex vein, ‘direct’ scleral invasion. Our observations were compared with the findings of the COMS report no. 6,16 the largest prospective series of uveal melanoma patients reported in the literature. Status with regard to liver metastasis and length of survival were ascertained using the departmental database, where this information is stored.
All samples included in this study were processed according to departmental protocols. Samples at enucleation were collected and fixed in 10% neutral buffered formalin for processing by the histopathology department. A macroscopic description of the eye was performed. The vortex veins were identified, assessed for the macroscopic presence of tumour, dissected off the main globe specimen by cutting along the lumen at the point of exit from the scleral canal and processed in a separate tissue cassette. Vortex veins were embedded longitudinally and ‘levels cut’ for microscopic assessment. In cases where the vortex veins had been cut flush with the sclera by the surgeon, the intra-scleral portion of the vein was removed by gently de-roofing the sclera, over the vein. The tissue was processed on a tissue processor using a standard overnight schedule. The tissue was passed through a series of graded alcohols, then through three changes of xylene and finally impregnated with paraffin wax, under vacuum. Once processed the tissue was embedded using standard techniques. Paraffin wax sections were cut using a rotary microtome and stained with haematoxylin and eosin. A periodic acid Schiff (PAS) stain was performed to identify the presence of extracellular networks and loops. All sections were reviewed by a single histopathologist.
Fluorescence in situ hybridization (FISH) for changes in the numbers of chromosomes 3 and 8: Archival analysis was performed on stored frozen chromosome preparations from short-term culture and tissue pieces. Prospective analysis was performed on newly prepared chromosome preparations, from both cultured and direct samples, made using well-established standard techniques.17 Chromosomes 3 and 8 α-satellite probes (labelled red and green, respectively) were used and supplied by Vysis. FISH analysis was performed according to standard protocols recommended by the manufacturers.17 For each case the numbers of signal per cell for chromosomes 3 and 8 were counted, and a total of 300 cells scored. Less signals for chromosome 3 in relation to chromosome 8 were considered to represent genetic imbalance (GI), a term encompassing various combinations of either loss or retention of chromosome 3, in relation to no change or gains of 8q.17
This study is descriptive in nature. The number of cases studies was judged to be small for a formal statistical assessment. All measurements such as tumour size were handled as continuous variables. Histopathological features were treated as categorical data. Differences in frequency of histopathological features were compared with the COMS16 study data using the χ2-statistic.
Results
Classification of vortex vein invasion
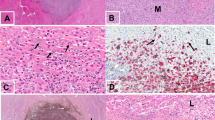
To be included in this study, uveal melanomas had to at least show evidence of microscopic intra-scleral vortex vein invasion (Figure 1a). Intra-scleral invasion was mostly identified in sections of whole eyeballs where the section passed through the intra-scleral course of the vortex veins. In our sample of 244 enucleated eyes that had a histological diagnosis of uveal melanoma, 29 eyes showed evidence of microscopic intra-scleral vortex vein invasion. Of these, 19 of 29 (65.5%) uveal melanomas were seen to have microscopic extra-scleral vortex vein involvement, whereas 10 of 29 (34.5%) did not. In some cases, the same vein that showed intra-scleral invasion, contained melanoma within the extra-scleral portion; in other cases, different veins showed intra-scleral invasion to those displaying extra-scleral invasion (not quantified).
Only 6 of 29 uveal melanomas (20.7%) were seen to have macroscopic evidence of vortex vein invasion at the pathology ‘cut-up’ bench (Figure 1b), whereas 23 of 29 (79.3%) had no macroscopic evidence of vortex vein invasion.
Direct invasion
Of the uveal melanomas that showed vortex vein invasion, 14 of 29 (48.3%) showed evidence of direct invasion (through scleral collagen, perineural or non-vortex vein perivascular invasion) whereas 15 of 29 (51.7%) did not.
Demographics
Analysis of demographic data of the vortex vein study group of patients revealed the mean age of patients to be 66.6 years (range 50–83 years), compared to the mean age in the COMS study of 60.1 years. A total of 15 (51.7%) of patients were male, and 14 (48.3%) were female. All patients were white Caucasian. The right eye was affected in 18 of 29 patients (62.1%) and the left eye in 11 of 29 (37.9%).
Tumour characteristics
Site
In our group of patients with vortex vein invasion, 23 of 29 (79.3%) of melanomas involved the choroid, 2 of 29 (6.9%) involved the ciliary body, and 4 of 29 (13.8%) involved both choroid and ciliary body (Figure 2a). Although the COMS study does not state the location of tumours that were found to have vortex vein invasion, it reports that 34.5% of these tumours partially involved the ciliary body. Comparing this figure to our data suggests that a choroidal location is overrepresented in cases of vortex vein invasion. Almost all uveal melanomas with vortex vein invasion had a peripheral location (Figure 2b).
Tumour characteristics of uveal melanomas showing vortex vein invasion. (a) Pie chart showing site of uveal melanomas with vortex vein invasion. (b) Pie chart demonstrating location of uveal melanomas with vortex vein invasion. (c) Scatter chart demonstrating maximum diameter and thickness of uveal melanomas with vortex vein invasion. (d) Pie chart showing the pattern of growth of uveal melanomas with vortex vein invasion.
Size
The mean maximum diameter of tumours with vortex vein invasion was 15.7 mm (range 10.3–20.8 mm) and the mean thickness was 9.7 mm (range 2.9–15.0 mm) (Figure 2c). Uveal melanomas have been classified as small (height <3 mm, largest basal diameter <10 mm), medium (height 3–8 mm, largest basal diameter <15 mm), or large (height 8 mm, largest basal diameter >15 mm).8 According to this classification, the mean maximum diameter and mean thickness of uveal melanomas with vortex vein invasion corresponds to a large tumour size.
Growth pattern
The uveal melanoma took the shape of a discrete nodule in 27 of 29 (93.1%) of cases with vortex vein invasion (Figure 2d).
Histopathological factors
Cell type
Of the uveal melanomas with vortex vein invasion, 19 of 29 (65.5%) were mixed cell (Figure 3a). However, 8 of 29 (27.6%) tumours were spindle cell (the term spindle encompassing ‘spindle A’ and ‘spindle B’ melanoma cells). Cell type was investigated in the COMS no. 6 study, which reports that 9% of medium and large uveal melanomas were spindle cell. Our results suggest that a spindle melanoma cell type is overrepresented in vortex vein invasion, when compared with the ‘general’ tumour population described in the COMS study (χ2=12.03, 2 d.f., P<0.05). The COMS study also states that 33.3% of medium tumours and 25.0% of large tumours of spindle cell type had vortex vein invasion, supporting our observation that spindle cell type is overrepresented in cases of vortex vein invasion.
Presence of extracellular matrix loops and networks
Our results reveal that 22 of 29 (75.9%) uveal melanomas with vortex vein invasion showed extracellular matrix loops and networks (Figure 3b). Datum for 1 of 29 uveal melanoma was not available.
Genetic imbalance by FISH
Uveal melanomas were classified to possess GI if they showed less signals for chromosome 3 centromere compared to those of chromosome 8 using (FISH).17 FISH data were available for 28 of 29 (96.6%) of uveal melanomas with vortex vein invasion. Of these, 25 of 29 (86.2%) showed GI.
Liver metastasis and survival
The mean follow-up interval for patients was 32.7 months (range 9–108 months).
In our group of patients with vortex vein invasion, 19 of 29 (65.5%) had radiologically confirmed liver metastasis. Of 29, 8 (27.6%) patients did not develop liver metastasis at the last known follow-up. The status of 2 of 29 (6.9%) of patients with regard to the presence of liver metastasis was unknown.
In patients with vortex vein invasion, 20 of 29 (69.0%) patients died, whereas 9 of 29 (31%) were alive at last known follow-up. Of the patients with vortex vein invasion who died, the cause of death was liver metastasis in 18 of 20 (90%) patients, with a mean survival of 31.9 months (range 9–108 months) from enucleation. In 2 of 20 (10%) of patients who died we were not able to confirm a cause of death. However, all 20 of the patients who died during the course of this study had GI according to FISH analysis, so it is probable that the 2 patients with an unknown cause of death also died from liver metastasis. One patient with confirmed liver metastasis is alive to date (survival length 27 months).
Orbital recurrence of uveal melanoma
There are no patients with uveal melanomas showing vortex vein invasion who have been found to show orbital recurrence of uveal melanoma following enucleation.
Discussion
This study has examined clinical, demographic, histopathological, and genetic factors in patients with vortex vein invasion in uveal melanoma, to establish whether vortex vein invasion correlates with identified prognostic factors in uveal melanoma. Our trends show that vortex vein invasion correlates with established indicators of poor prognosis. The exception is the overrepresentation of spindle cell type in uveal melanomas with vortex vein invasion.
Risk factors for vortex vein invasion
This study implicates the site of the uveal melanoma as a risk factor for vortex vein invasion. Our results suggest that a choroidal location is overrepresented in cases of vortex vein invasion when compared with the results of the COMS report no.6,16 which provides data on a large series of uveal melanomas. Classically, ciliary body melanomas have been associated with a poorer outlook,6 but ciliary body tumours are less commonly seen in our patients with vortex vein invasion. The reason for this is that ciliary body tumours are further from the vortex veins, and so vortex vein invasion is not as likely from an anatomical viewpoint. Only 2 of 29 uveal melanomas with vortex vein invasion had a posterior pole location. This suggests a peripheral location is a risk factor for vortex vein invasion, which also corresponds to the anatomical location of the vortex veins.
As one would expect, a greater maximum diameter and thickness of tumour at the time of diagnosis correlate with a poorer outcome.8 Uveal melanomas are often classified as small, medium, or large.8 The mean maximum diameter and thickness of tumours in our patients with vortex vein invasion corresponds to a ‘large’ tumour size. This suggests that size of tumour is a risk factor for vortex vein invasion, although it is acknowledged that large tumours can metastasize independent of vortex vein involvement.8
The association of vortex vein invasion with tumour cell type in our study yields conflicting results, as a spindle cell type is overrepresented. This also appears to be the case in the subset of patients with vortex vein invasion in the COMS report no. 6.16 However, spindle cell type is associated with a better outcome when compared with mixed or epithelioid tumours.9 This association may be due to our small sample size. To address the issue of whether there is a subset of spindle-rich melanomas with aggressive behaviour is beyond the scope of this descriptive study.
The presence of extracellular matrix loops and networks in uveal melanomas is associated with a poorer outcome.10, 11 Our data suggest the presence of extracellular matrix loops and networks is a risk factor for vortex vein invasion, as 22 of 29 (75.9%) of cases of vortex vein invasion contained extracellular matrix loops and networks.
GI is another risk factor for vortex vein invasion, seen in 25 of 29 patients in this study.
This study did not address whether uveal melanomas with vortex vein invasion also showed retinal invasion. In our centre, the ocular oncologists routinely perform a sampling sclerotomy, straight after the enucleation, remove retina over the tumour, and sample the tumour for cytogenetics. As the retina overlying the tumour is compromised, it was not possible for us to accurately assess retinal invasion.
Vortex vein invasion and survival
All patients included in this study had a minimum of microscopic intra-scleral vortex vein invasion. However, only 6 of 29 (20.7%) of patients had macroscopic vortex vein invasion. Patients who showed only microscopic intra-scleral vortex vein invasion did not have a better outcome, as 9 of 10 patients died during the follow-up period, 7 from confirmed liver metastasis. The cause of death in the remaining 2 patients was not confirmed, but given that both these patients had GI by FISH testing, liver metastasis is a strong possibility. This underlines the importance of microscopic assessment of vortex veins for invasion by tumour, and indicates that a macroscopic examination of the vortex veins at the pathology ‘cut-up’ bench is insufficient. Our results also suggest vortex vein invasion occurs independently of ‘direct’ non-vortex vein scleral invasion (peri-vascular, peri-neural, or by the scleral collagen), as illustrated by the fact that approximately half of our patients with vortex vein invasion had no evidence of direct extra-ocular invasion.
The data suggest that patients with vortex vein invasion appear to have a worse prognosis, with 69.0% of patients developing liver metastasis, at the most recent follow-up. Although it is acknowledged that ‘large’ tumours have a higher metastatic rate,8 independent of vortex vein invasion, the question that must be asked is why ‘large’ tumours have a higher metastatic rate. This observation is not peculiar to uveal melanoma; generally, the larger the malignant tumour (of whatever histogenesis) the higher the metastatic rate. Large tumours encroach upon vascular beds and invade these vascular channels leading to metastasis. Therefore the probability of seeing tumour in vortex veins draining a ‘large’ tumour is not surprising. This may or may not be independent of other tumour characteristics, such as cytology or the presence of extracellular matrix loops or networks.
The average survival of patients with liver metastasis was 31.9 months. There were 8 patients with vortex vein invasion that did not develop liver metastasis at the time of last follow-up. However, it is difficult to give accurate data regarding the survival of these patients because the follow-up data available was short and the sample size small. Given that we receive referrals to the ocular oncology unit from across the UK, patients sometimes elect to have local follow-up once they have received surgical treatment. In this situation, we are reliant on the local hospital responding to our inquiries regarding patient outcome. It is likely that some of these patients have gone on to develop liver metastasis but that we were not informed. However, at least one patient in the no metastasis group has survived a period of 106 months, but this is the only patient known to have reached the 5-year survival period in this group. Given the observed trend of vortex vein invasion with liver metastasis and GI, it is possible that invasion of the vortex veins is an important route through which uveal melanoma accesses the bloodstream with haematogenous spread and subsequent liver metastasis.
Impact of the study on the handling of enucleations for melanoma
The ophthalmic pathology practice in Sheffield has been to sample all vortex veins at the pathology ‘cut-up’ bench. This has involved sampling obvious lengths of extra-ocular portions of vortex veins and intra-scleral portions of veins (in cases where the veins have been cut flush with the sclera during the enucleation). The veins are assessed with the naked eye for the presence or absence of tumour. Our practice has been to embed the vortex veins longitudinally and to cut several levels through them to look for microscopic involvement by melanoma. This study has shown that there are cases where the vortex vein shows no macroscopic evidence of tumour, yet show microscopic evidence of tumour in the vein lumen. As this study suggests a correlation between vortex vein invasion and other well-established prognostic factors, vortex veins will be continued to be sampled and examined in this way.
Vortex vein invasion is associated with a poor outcome in patients with uveal melanoma. Invasion of vortex veins correlates with other poor prognostic factors.
References
Ries LAG, Eisner MP, Kosary CL et al. SEER cancer statistics review, 1973–1999. National Cancer Institute. Bethesda, MD http://seer.cancer.gov/csr/1973_1999/, 2002.
Shields JA, Shields CL, Donoso LA . Management of posterior uveal melanoma. Surv Ophthalmol 1991; 36: 161–195.
COMS. Assessment of metastatic disease status at death in 45 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol 2001; 119: 670–676.
Kath R, Havungs J, Bornfield N, Sauerwein W, Hoffken K, Seeber S . Prognosis and treatment of disseminated uveal melanoma. Cancer 1993; 72: 2219–2223.
Gragoudos ES, Egan KM, Seddon JM, Glynn RJ, Walsh SM, Finn SM et al. Survival of patients with metastases from uveal melanoma. Ophthalmology 1991; 98: 383–389.
Seddon JM, Albert DM, Lavin PT, Robinson N . A prognostic factor study of disease-free interval and survival following enucleation for uveal melanoma. Arch Ophthalmol 1983; 101: 1894–1899.
Shields CL, Shields JA, Materin M, Gershbaum E, Singh AD, Smith A . Iris melanoma: risk factors in 169 consecutive cases. Ophthalmology 2001; 108: 172–178.
Diener-West M, Hawkins BS, Markowitz JA, Schachat AP . A review of mortality from choroidal melanoma. II. A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988. Arch Ophthalmol 1992; 110: 245–250.
McLean IW, Foster WD, Zimmermann LE . Uveal melanoma: location, size, cell type, and enucleation as risk factors in metastasis. Hum Pathol 1982; 13: 123–132.
Folberg R, Pe’er J, Grumn LM, Woolson RF, Jeng G, Montague PR et al. The morphologic characteristics of tumour blood vessels as a marker of tumour progression in primary human uveal melanoma: a matched case-control study. Hum Pathol 1992; 23: 1298–1305.
Folberg R, Rummelt V, Parys-Van Ginderdeuren R, Hwang T, Woolson RF, Pe’er J et al. The prognostic value of tumour blood vessel morphology in primary uveal melanoma. Ophthalmology 1993; 100: 1389–1398.
Seddon JM, Polivogianis L, Hsieh CC, Albert DM, Gamel JW, Gragoudas ES . Death from uveal melanoma. Number of epithelioid cells and inverse SD of nucleolar area as prognostic factors. Arch. Ophthalmol. 1987; 105: 801–806.
McGurdy J, Gamel J, McClean I . A simple, efficient, and reproducible method for predicting the malignant potential of uveal melanoma for routine H & E slide. Pathol Res Pract 1991; 187: 1025–1027.
Prescher G, Bornfold N, Hirche H, Horsthemke B, Karl-Heinz J, Becher R . Prognostic implications of monosomy 3 in uveal melanoma. Lancet 1996; 347: 1222–1225.
White VA, Chambers JD, Courtright PD, Chang WY, Horsman DE . Correlation of cytogenetic abnormalities with the outcome of patients with uveal melanoma. Cancer 1998; 83: 354–359.
COMS. Histopathologic characteristics of uveal melanoma in eyes enucleated from the Collaborative Ocular Melanoma Study COMS report no. 6. Am J Ophthalmol 1998; 125: 745–766.
Patel KA, Edmondson ND, Talbot F, Parsons MA, Rennie IG, Sisley K . Prediction of prognosis inpatients with uveal melanoma using fluorescence in situ hybridization. Br J Ophthalmol 2001; 85: 1440–1444.
Acknowledgements
This work was in part supported by Trent Regional Health RBF00XX5 and Yorkshire Eye Research (003). Figures were prepared with assistance from Mr Robin Farr, Senior Technician, Academic Unit of Ophthalmology and Orthoptics, University of Sheffield.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/Conflict of interest
The author has no financial interest in any of the products mentioned.
Rights and permissions
About this article
Cite this article
Raoof, N., Rennie, I., Salvi, S. et al. What is the significance of vortex vein invasion in uveal melanoma?. Eye 23, 1661–1667 (2009). https://doi.org/10.1038/eye.2008.345
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2008.345