Abstract
Aim
To study the solubility of perfluorohexyloctane (F6H8) in silicone oil (polydimethylsiloxane (PDMS) 1000) and to measure the viscosity and the specific gravity of the mixture obtained (heavy silicone oil or HSO tamponade) to define the ideal ratio of these components.
Methods
The solubility diagram of the mixture was obtained with the turbidimetric method, indicating the miscibility of F6H8 and silicone oil 1000 at all the useful temperatures. The viscosity was measured in steady shear conditions by using a controlled stress rheometer (Haake RS150) and a double cone/plate (DC 60/4) system, both at 25 and 37°C for different volume per cent compositions of the mixture. The specific gravity was measured at 37°C using a digital densimeter.
Results
A mixture of F6H8 30 v% and PDMS 70 v% was found to be transparent and stable at all the useful temperatures. By combining these proportions of the two substances, a resultant density of 1.06 g/cm3 was obtained. The viscosity of the 30% F6H8 mixture was 203 mPa.s at 25°C and 163 mPa.s at 37°C respectively.
Conclusions
The ideal F6H8 and silicone oil mixture can be obtained combining 30% of F6H8 with 70% of silicone oil 1000. This mixture seems to have rheological properties useful for its use as an alternative intraocular heavy tamponade.
Similar content being viewed by others
Introduction
Silicone oil has been shown to be effective as internal tamponade agent for the management of complicated rhegmatogenous retinal detachment especially those complicated by proliferative vitreoretinopathy (PVR).1, 2, 3, 4 Both gas and silicone oil have a specific gravity lower than that of water and thus are indicated when the tamponading effect is required in the upper quadrants. Recurrence of inferior retinal detachment is a frequent complication after silicone oil tamponade. Vitreoretinal proliferation occurs more frequently in the inferior quadrants, as cells gather in the watery space between the retina and the oil bubble due to the gravity.5, 6, 7, 8, 9 Therefore, there may be a specific role for tamponade agents with a specific gravity higher than that of water. Several heavier-than-water fluorinated silicone oils have been evaluated for intraocular use. Fluorinated silicone oil was introduced as an internal tamponade in the 1990s but its use was abandoned because of a high frequency of complications.10, 11, 12, 13, 14, 15, 16, 17 Perfluorocarbon liquids (PFCLs) have been introduced as tools in vitreoretinal surgery and then followed their indication as heavy tamponades.18, 19, 20 Nevertheless, it has been demonstrated that PFCL might not be well-tolerated as long-term internal tamponades due to retinal toxicity.21, 22, 23
Semifluorinated alkanes (FALKs) have first been introduced as solvents for silicone oil, and then this new group of substances has been proposed as long-term internal heavy tamponade.
Although they seem to be better tolerated than other heavy internal tamponades, their use is complicated by their frequent emulsification and small bubble dispersion.24, 25, 26, 27, 28
Recently, the use of silicone oil in combination with partially fluorinated alkanes has been proposed to obtain a heavy silicone oil.29, 30 The aim of our study was to investigate the solubility of perfluorohexyloctane (F6H8) in silicone oil (polydimethylsiloxane (PDMS) 1000) and to evaluate the viscosity and the specific gravity of the mixture obtained.
Materials and methods
The solubility of F6H8 in PDMS 1000, the viscosity and the specific gravity of the mixtures were investigated.
The capacity of two substances to dissolve into one another depends on concentration and temperature. At a given temperature and pressure, a mixture of two substances can result in either a homogeneous or a two-phase system, with a mixing ratio determined by the type of components. Typically for two liquid substances mixed together, at any given temperature, it is possible to recognize a miscibility gap, which is a composition range where the two liquids do not mix to give a homogeneous solution, but form two separate solutions or phases having different compositions. In most cases, an increase of temperature causes an increase of solubility, and above a given temperature, the mixture is homogeneous at any composition.31
We determined the solubility diagram of F6H8 (Fluoron GmbH, Neu Ulm, Germany) in PDMS 1000 mPa.s (Fluoron GmbH) by means of the turbidimetric method. This method is based on the principle according to which raising the temperature increases the solubility of the two components. The temperature at which turbidity or opacity of the mixture disappeared, during heating and under stirring, was determined at each composition. The temperature of opacity appearance during cooling was then detected. The mean of the two temperature values, which were usually close (0.1–0.2°C), was the critical temperature of the solution. This value was inserted in a graphic with the percentage of F6H8 in abscissa.
We then evaluated the viscosity of the F6H8-PDMS mixture for different percentage of composition. Viscosity measurements were performed in steady shear conditions by using a controlled stress rheometer (Haake RS150) and a double cone/plate (DC 60/4) system. The viscosity values were performed at both 25 and 37°C for mixtures of F6H8-PDMS at different volume per cent compositions (v%). The values of v%, which is more familiar to the surgeon than mass per cent (m%), were calculated from m% using density values of pure liquids.
Finally, we ascertained the specific gravity of the mixture, which was measured at 37°C, the temperature at which the mixture has to produce the tamponade effect. To this purpose, we used a digital densimeter (Anton Paar DMA 602H—DMA 60) with a resolution of 1 × 10–5g/cm3. The density determination is based on measuring the period of oscillation of a vibrating U-shaped tube filled with the sample. The densitometer was calibrated using water and air.
Results
In Figure 1, the solubility diagram of the F6H8–PDMS 1000 mixture is represented. The critical solution temperatures are plotted against the v% for the F6H8–PDMS 1000 mixture. The curve separates the area where the mixture is uniform, homogeneous, and transparent from the area where the two components are not completely miscible and remain separated in two phases. At a given temperature, for instance 37°C, any mixture containing more than F6H8 60 v% will be splitted in two different phases: a solution with F6H8 60 v% and a solution with more than F6H8 99.5 v%, that is practically pure F6H8.
Solubility curve for the mixture F6H8–PDMS 1000. The critical solution temperature is plotted against the volume composition (v%) for the F6H8–PDMS 1000 mixture. The curve separates the area where the mixture is uniform, homogeneous and transparent (single-phase) from the area where the two components are not completely miscible (two phases).
The temperatures at which the mixture will be exposed range from 20°C outside the eye to 37°C and perhaps less inside the eye. The mixture to be used needs to have a critical solution temperature lower than 20°C, namely, it has to contain less than 36 v% of F6H8. On the other hand, to be heavier than water this mixture has to contain more than 10 v% of F6H8, which corresponds to a density of 1.00 g/cm3 (Table 1).
A mixture with F6H8 30 v% and PDMS 70 v% was chosen as a suitably heavy solution stable at all the useful temperatures.
Table 1 collects the viscosity values at both 25 and 37°C for mixtures of F6H8–PDMS at different v%. The viscosity of the 30% F6H8 mixture is 203 mPa.s at 25°C and 163 mPa.s at 37°C respectively. This heavy silicone oil has a density of 1.061 g/cm3.
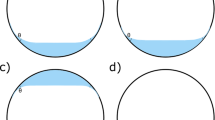
The Figure 2 shows how such a mixture appears before and after complete mixing. For comparison, the Figure 3 shows what happens for a mixture of F6H8 70 v% and PDMS 30 v%, that is, with a composition inside the immiscibility range.
Mixture of F6H8 70 v% and PDMS 1000 30 v%. A mixture of F6H8 70 v% and PDMS 1000 30 v% before (a), during (b), and after (c) shaking. Two separated phases become a cloudy mixture during mixing, and then two separated clear solutions in equilibrium with each other. (a) F6H8 and silicone oil. (b) Cloudy mixture. (c) Clear separate phases.
Discussion
The ideal vitreous substitute should tamponade the retina in all quadrants and has to be compatible with ocular tissues. Silicone oil and long-acting gases have a specific gravity lower than that of water and thus cannot support the inferior retina. For this reason, a heavier-than-water tamponade would be an extremely useful tool in preventing the recurrence of retinal detachment, which occurs mainly in the inferior quadrants after silicone oil tamponade.32
The first attempts made use of fluorinated silicone oil. This tamponade showed poor ocular tolerance because of its tendency to emulsify and to lead to severe intraocular inflammation and epiretinal membrane formation.10, 11, 16
Perfluorocarbon liquids, introduced by Chang as stronghold intraoperative tool, were considered to be used as long-term postoperative tamponades.18, 33, 34 However, several experimental and clinical studies demonstrated their ocular toxicity, showing that they caused significant damage to the retina, possibly on account of the high specific gravity of these substances, which exerted mechanical pressure on the retina.23, 35 In addition, a strong inflammatory response was observed. Eckardt et al and Elsing et al noticed the appearance of white proteinaceous deposits and macrophage activation in eyes with retained PFCL.22, 36
A new category of partially fluorinated alkanes has recently been introduced.27 These substances have a lower specific gravity than other PFCLs. According to the hypothesis that tissue damage is mainly related to the mechanical pressure they exert on the retina, this characteristic should minimize retinal injury. For this reason, they have been proposed as suitable materials for long-standing intraocular tamponade. The first experimental trial in rabbits revealed histological alterations of the inferior retina similar to those observed after the use of PFCL, suggesting that specific gravity is not the major factor in retinal damage. Furthermore, droplet formation was noticed as occurs with PFCL. Clinical findings in small series have suggested that F6H8 deserves consideration as an internal tamponade for extended periods. Nevertheless, the most common complication noted is the dispersion of this tamponade into droplets. This is one of the main concerns because the dispersion reduces the volume of the tamponade agent, thus reducing the contact with the surface of the retina.26, 37, 38
Hydrofluorocarbon oligomers, produced by joining two to four FALK molecules, have been developed to increase oligomer viscosity and to minimize dispersion. The OL62HV oligomer has a viscosity of 1750 mPa.s. Experimental use of this tamponade in rabbits showed no droplet formation and a better preservation of retinal vascular architecture.39 These experimental results lead the authors of this research to conclude that this oligomer was well-tolerated and a promising candidate for use as a tamponade.
Roider et al26 tested FALK (perfluorohexyloctane) and a high-viscosity FALK oligomer (OL62HV) in a small clinical series. They observed in both groups an unusual biological reaction not shown up in experimental trials, such as fluffy epiretinal membranes with cystic cells and amorphous material mainly located on the surface of the tamponade. The use of high-viscosity oligomer did not reveal droplet formation. Two cases occurred of retinal necrosis with constricted retinal vessels and one case of atrophy of the optic nerve. The authors suspended the trial concluding that this oligomers are not tolerated by the human use, raising questions regarding the suitability of the rabbit model for testing the tolerance of new substances.26
In a clinicopathological report, Vote et al suggested that F6H8 had a proinflammatory effect, possibly related to its tendency to disperse.40 In addition, recent experimental studies have pointed to a possible toxic effect of F6H8 on animal and human cell cultures.41, 42 From the foregoing, it is clear that the use of FALK as internal tamponade is still a concern. Owing to their amphilic nature, FALKs are soluble in oil and liquid perfluorocarbon but insoluble in water.27 Thanks to this characteristic F6H8 was initially introduced as a silicone solvent to eliminate small droplets after silicone oil removal.25, 27
Recently, the possibility of producing a solution of FALK and silicone oil has led to the proposal to use mixtures of these two substances as heavy tamponades. The idea of combining two tamponades emerged in the 1990s, when a simultaneous double-filling blending fluorosilicone and silicone oil was proposed to avoid free spaces developing in the vitreous cavity. The resulting mixture was a two-bubble compound with a high rate of reproliferation. The reproliferation occurred especially along a tamponade-free horizontal line involving the posterior pole and corresponding to the interface between the two bubbles.16, 43
The combination of FALK and silicone oil could result in a single-bubble optically clear mixture, according to the proportions of the two components. This would provide a real ‘heavy silicone oil’.27, 29, 30 Our experimental results show that the addition of 30 v% of F6H8 to silicone oil 1000 mPa.s results in a clear and homogeneous mixture at temperatures higher than 10°C (Figure 1). We chose this percentage of F6H8 for two main reasons. First, to obtain a mixture stable and transparent both at storage and body temperature, which is essential for a surgical purpose. Second, to obtain a high enough density (1.061 g/cm3) ensuring the tamponade effect on the inferior retina.44
The choice of using 1000 mPa.s instead of 5000 mPa.s silicone oil also leads to further considerations. Hoerauf and colls found that an addition of 30 v% of F6H8 to silicone oil (5000 mPa.s) produced a mixture with a specific gravity of 1.08 g/ml and a viscosity of 1136 mPa.s.29 Meinert and Roy27 observed that the solubility of FALK in silicone oil increases as the viscosity of silicone oil decreases from 5000 to 1000 mPa.s. As a consequence, a more stable mixture can be obtained using 1000 mPa.s silicone oil.
Two heavy silicone oils, Oxane HD (Bausch&Lomb Inc., Waterford, Ireland) and Densiron (Fluoron GmbH), have recently been launched in the European market. Both are mixtures of silicone oil 5000 mPa.s and FALK: Oxane HD contains 10.2 v% of a partially fluorinated olefin (RMN3), whereas Densiron 68 contains 30 v% of F6H8.
Using the 1000 mPa.s silicone oil, the viscosity of the 30% F6H8 mixture is 203 mPa.s at 25°C and 163 mPa.s at 37°C. This low viscosity facilitates the injection of the mixture, which can be performed either with active pump or manually with a syringe and a dual-lumen cannula, enabling the mixture to be injected in a similar manner to that of PFCL. Furthermore, a mixture of low viscosity and relatively high specific density will reduce the loss of tamponade close to scleral bucklings.30, 45
The only concern arising from the utilization of low-viscosity mixtures is their possible tendency to disperse. It is well known that the factor mainly involved in dispersion is the interfacial tension. This expresses the forces that tend to hold molecules together when two non-miscible liquids are in contact. The higher the interfacial tension, the lower the tendency to disperse.46 The interfacial tension against water of silicone oil 1000 mPa.s at 25°C ranges from 23.3 to 35.4 mN/m according to different authors and that of silicone oil 5000 mPa.s is reported to be 35.4 mN/m;27, 47, 48, 49 the interfacial tension of F6H8 is 49.1 mN/m, which is higher than both silicone oils. Nevertheless, F6H8 tends to disperse more than silicone oils. This is mainly due to the different viscosities of these substances, the viscosity of F6H8 being 2.5 mPa.s.29 Viscosity does not affect the interfacial tension, but a highly viscous substance does not disperse as quickly as a mildly viscous one.46 Dispersion is influenced by mechanical energy applied to the tamponade. Thus, increasing viscosity increases the time needed to develop dispersion, by increasing the resistance of the tamponade to the mechanical intraocular shaking.50
For this reason, many surgeons prefer to use 5000 cs instead of 1000 cs. However, controlled clinical trials comparing the propensity of these two oils to emulsify do not exist as yet. In addition, it should be stressed that from a physical point of view the high ratio between the oil and the water phases makes oil emulsification unlikely, this ratio being far from phase reversal. Under these conditions, what ensures high internal phase ratio of emulsions is the presence of contaminants acting as surfactants. This is a crucial point. Many substances may perform this fundamental role, such as blood constituents, lipoproteins, phospholipids, oligosiloxanes, perfluorocarbons, and metal ions. These substances may reduce the interfacial tension and greatly facilitate the emulsification.51, 52, 53, 54
A recent study by Dresp and Menz55 demonstrated a great variety of contaminants in emulsified samples of explanted silicone oil, when compared with non-emulsified samples. This was found in both 1000 and 5000 cs samples.
Another important factor involved in the emulsification is the duration of the tamponade. It is a common clinical experience that the longer the tamponade, the greater the emulsification.56
In conclusion, we believe that silicone oil emulsification should be considered a multifactorial event, varying in frequency and severity according to patient conditions, surgical technique, and tamponade duration.
Heavy silicone oils show interfacial tension values similar to standard silicone oils. The interfacial tension against water of a mixture of silicone oil 1000 mPa.s with 42 v% of F6H8 is 26.6 mN/m.57 This value is similar to that of standard silicone oils and is very different from that of F6H8 (49.1 mN/m). A similar behaviour was observed for the mixture of F6H8 and silicone oil 5000 mPa.s.27 Thus, these mixtures seem to behave more like the silicone oil than F6H8, the interfacial tension of silicone oil being not particularly influenced by adding F6H8 30 v%.
A mixture of silicone oil 5000 mPa.s with 33% (w/w) F6H8 has a viscosity of 1275 mPa.s,27 and our mixture of silicone oil 1000 mPa.s with F6H8 30 v% exhibits a viscosity of 164 mPa.s (at 37°C) indicating that the addition of F6H8 to silicone oil markedly reduces its viscosity.30 This mixture has been successfully tested in a prospective consecutive interventional case series study including patients with inferior retinal detachment complicated by severe PVR and inferior retinal tears.58 In the present study the mixture was created by our staff in the surgical room but it has been available on the market (Densiron 68 LV - Fluoron GmbH) for a few months.
In conclusion, the ideal F6H8 and silicone oil mixture is obtained combining 30% of F6H8 with 70% of silicone oil 1000. Infact with these ratios, we obtain a heavy silicone oil stable at all the useful temperatures and with a specific gravity that makes it suitable for inferior tamponade. Furthermore, its rheological properties make this mixture far more manageable than the silicone oil 1000.
References
McCuen III BW, de Juan Jr E, Machemer R . Silicone oil in vitreoretinal surgery. Part 1: Surgical techniques. Retina 1985; 5: 189–197.
McCuen II BW, de Juan Jr E, Landers III MB, Machemer R . Silicone oil in vitreoretinal surgery. Part 2: Results and complications. Retina 1985; 5: 198–205.
Sell CH, McCuen II BW, Landers III MB, Machemer R . Long-term results of successful vitrectomy with silicone oil for advanced proliferative vitreoretinopathy. Am J Ophthalmol 1987; 103: 24–28.
Hammer ME, Rinder DF, Hicks EL, Yang CH, Hornung CA . Tolerance of perfluorocarbons, fluorosilicone and silicone liquids in the vitreous. In: Freeman, Tolentino (eds). Proliferative Vitreoretinopathy. Springer-Verlag: New York, 1988, pp 156–161.
Sharma T, Gopal L, Shanmugam MP, Bhende PS, Agrawal R, Badrinath SS et al. Management of recurrent retinal detachment in silicone oil-filled eyes. Retina 2002; 22: 153–157.
Falkner CI, Binder S, Kruger A . Outcome after silicone oil removal. Br J Ophthalmol 2001; 85: 1324–1327.
Zilis JD, McCuen II BW, de Juan Jr E, Stefansson E, Machemer R . Results of silicone oil removal in advanced proliferative vitreoretinopathy. Am J Ophthalmol 1989; 108: 15–21.
Riedel KG, Gabel VP, Neubauer L, Kampik A, Lund OE . Intravitreal silicone oil injection: complications and treatment of 415 consecutive patients. Graefes Arch Clin Exp Ophthalmol 1990; 228: 19–23.
Abrams GW, Azen SP, McCuen II BW, Flynn Jr HW, Lai MY, Ryan SJ . Vitrectomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy: results of additional and long-term follow-up. Silicone Study report 11. Arch Ophthalmol 1997; 115: 335–344.
Gabel VP, Kampik A, Gabel C, Spiegel D . Silicone oil with high specific gravity for intraocular use. Br J Ophthalmol 1987; 71: 262–267.
Nakamura K, Refojo MF, Crabtree DV, Pastor J, Leong FL . Ocular toxicity of low-molecular-weight components of silicone and fluorosilicone oils. Invest Ophthalmol Vis Sci 1991; 32: 3007–3020.
Friberg TR, Verstraeten TC, Wilcox DK . Effects of emulsification, purity, and fluorination of silicone oil on human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 1991; 32: 2030–2034.
Gremillion Jr CM, Peyman GA, Liu KR, Naguib KS . Fluorosilicone oil in the treatment of retinal detachment. Br J Ophthalmol 1990; 74: 643–646.
Eckardt C, Schmidt D, Czank M . Intraocular tolerance to silicone oils of different specific gravities. An experimental study. Ophthalmologica 1990; 201: 133–139.
Peyman GA, Smith RT, Charles H . Injection of fluorosilicone oil and pars plana vitrectomy for complex retinal detachment. Can J Ophthalmol 1987; 22: 276–278.
De Molfetta V, Bottoni F, Arpa P, Vinciguerra P, Zenoni S . The effect of simultaneous internal tamponade on fluid compartmentalization and its relationship to cell proliferation. Retina 1992; 12: S40–S45.
Pastor JC, Lopez MI, Saornil MA, Refojo MF . Intravitreal silicone and fluorosilicone oils: pathologic findings in rabbit eyes. Acta Ophthalmol 1992; 70: 651–658.
Chang S, Ozmert E, Zimmerman NJ . Intraoperative perfluorocarbon liquids in the management of proliferative vitreoretinopathy. Am J Ophthalmol 1988; 106: 668–674.
Verma LK, Peyman GA, Wafapoor H, Greve MD, Millsap CM, Adile SL . An analysis of posterior segment complications after vitrectomy using the perfluorocarbon perfluoroperhydrophenanthrene (Vitreon). Vitreon Collaborative Study. Ophthalmic Surg 1995; 26: 29–33.
Blinder KJ, Peyman GA, Desai UR, Nelson Jr NC, Alturki W, Paris CL . Vitreon, a short-term vitreoretinal tamponade. Br J Ophthalmol 1992; 76: 525–528.
Eckardt C, Nicolai U . Clinical and histologic findings after several weeks of intraocular tamponade with perfluorodecalin. Ophthalmologe 1993; 90: 443–447.
Eckardt C, Nicolai U, Winter M, Knop E . Experimental intraocular tolerance to liquid perfluorooctane and perfluoropolyether. Retina 1991; 11: 375–384.
Velikay M, Wedrich A, Stolba U, Datlinger P, Li Y, Binder S . Experimental long-term vitreous replacement with purified and nonpurified perfluorodecalin. Am J Ophthalmol 1993; 116: 565–570.
Langefeld S, Kirchhof B, Meinert H, Roy T, Aretz A, Schrage NF . A new way of removing silicone oil from the surface of silicone intraocular lenses. Graefes Arch Clin Exp Ophthalmol 1999; 23: 201–206.
Dick HB, Augustin AJ . Solvent for removing silicone oil from intraocular lenses: experimental study comparing various biomaterials. J Cataract Refract Surg 2000; 26: 1667–1672.
Roider J, Hoerauf H, Kobuch K, Gabel VP . Clinical findings on the use of long-term heavy tamponades (semifluorinated alkanes and their oligomers) in complicated retinal detachment surgery. Graefes Arch Clin Exp Ophthalmol 2002; 240: 965–971.
Meinert H, Roy T . Semifluorinated alkanes-a new class of compounds with outstanding properties for use in ophthalmology. Eur J Ophthalmol 2000; 10: 189–197.
Kirchhof B, Wong D, Van Meurs J, Hilgers RD, Macek M, Lois N et al. Use of perfluorohexyloctane as a long-term internal tamponade agent in complicated retinal detachment surgery. Am J Ophthalmol 2002; 133: 95–101.
Hoerauf H, Kobuch K, Dresp J, Menz DH . Combined use of partially fluorinated alkanes, perfluorocarbon liquids and silicone oil: an experimental study. Graefes Arch Clin Exp Ophthalmol 2001; 239: 373–381.
Herbert E, Stappler T, Wetterqvist C, Williams R, Wong D . Tamponade properties of double-filling with perfluorohexyloctane and silicone oil in a model eye chamber. Graefes Arch Clin Exp Ophthalmol 2003; 242: 250–254.
Novak JP, Matous J, Pick J . Liquid–Liquid Equilibria. Elsevier: Amsterdam, 1987.
Singh AK, Glaser BM, Lemor M, Michels RG . Gravity-dependent distribution of retinal pigment epithelial cells dispersed into the vitreous cavity. Retina 1986; 6: 77–80.
Chang S, Zimmerman NJ, Iwamoto T, Ortiz R, Faris D . Experimental vitreous replacement with perfluorotributylamine. Am J Ophthalmol 1987; 103: 29–37.
Peyman GA, Schulman JA, Sullivan B . Perfluorocarbon liquids in ophthalmology. Surv Ophthalmol 1995; 39: 375–395.
Chang S, Sparrow JR, Iwamoto T, Gershbein A, Ross R, Ortiz R . Experimental studies of tolerance to intravitreal perfluoro-n-octane liquid. Retina 1991; 11: 367–374.
Elsing SH, Fekrat S, Green WR, Chang S, Wajer SD, Haller JA . Clinicopathologic findings in eyes with retained perfluoro-n-octane liquid. Ophthalmology 2000; 108: 45–48.
Stefaniotou MI, Aspiotis MV, Kitsos GD, Kalogeropoulos CD, Asproudis IC, Psilas KG . Our experience with perfluorohexyloctane (F6H8) as a temporary endotamponade in vitreoretinal surgery. Eur J Ophthalmol 2002; 12: 518–522.
Wong D, Lois N . Perfluorocarbons and semifluorinated alkanes. Semin Ophthalmol 2000; 15: 25–35.
Kobuch K, Menz IH, Hoerauf H, Dresp JH, Gabel VP . New substances for intraocular tamponades: perfluorocarbon liquids, hydrofluorocarbon liquids and hydrofluorocarbon-oligomers in vitreoretinal surgery. Graefes Arch Clin Exp Ophthalmol 2001; 239: 635–642.
Vote B, Wheen L, Cluroe A, Teoh H, McGeorge A . Further evidence for proinflammatory nature of perfluorohexyloctane in the eye. Clin Exp Ophthalmol 2003; 31: 408–414.
Mertens S, Bednarz J, Engelmann K . Evidence of toxic side effects of perfluorohexyloctane after vitreoretinal surgery as well as in previously established in vitro models with ocular cell types. Graefes Arch Clin Exp Ophthalmol 2002; 240: 989–995.
Malchiodi-Albedi F, Matteucci A, Formisano G, Paradisi S, Carnovale-Scalzo G, Perilli R et al. Perfluorohexyloctane (F6H8) induces structural modifications and increases apoptosis in rat primary retinal cultures. J Biomed Mater Res 2003; 15: 133–136.
Bottoni F, Arpa P, Vinciguerra P, Zenoni S, De Molfetta V . Combined silicone and fluorosilicone oil tamponade (double filling) in the management of complicated retinal detachment. Ophthalmologica 1992; 204: 77–81.
Peyman GA, Conway MD, Soike KF, Clark Jr LC . Long-term vitreous replacement in primates with intravitreal Vitreon or Vitreon plus silicone. Ophthalmic Surg 1991; 22: 657–664.
Williams R, Wong D . The influence of explants on the physical efficiency of tamponade agents. Graefes Arch Clin Exp Ophthalmol 1999; 237: 870–874.
de Juan Jr E, McCuen B, Tiedeman J . Intraocular tamponade and surface tension. Surv Ophthalmol 1985; 30: 47–51.
Lepori L, Matteoli E, Spanedda A, Genovesi-Ebert F, Rizzo S . Combined use of perfluorohexyloctane and silicone oil as intraocular tamponade: an in vitro study. Graefes Arch Clin Exp Ophthalmol 2006; 244: 79–82.
Wetterqvist C, Wong D, Williams R, Stappler T, Herbert E, Freeburn S . Tamponade efficiency of perfluorohexyloctane and silicone oil solutions in a model eye chamber. Br J Ophthalmol 2004; 88: 692–696.
Wong D, Kumar I, Quah SA, Ali H, Valdeperas X, Romano MR . Comparison of postoperative intraocular pressure in patients with Densiron-68 vs conventional silicone oil: a case–control study. Eye advance online publication 7 December 2007; doi:10.1038/sj.eye.6703055.
Francis JH, Latkany PA, Rosenthal J . Mechanical energy from intraocular instruments cause emulsification of silicone oil. Br J Ophthalmol 2007; 91: 818–821.
Wickham LJ, Asaria RH, Alexander R, Luthert P, Charteris DG . Immunopathology of intraocular silicone oil: retina and epiretinal membranes. Br J Ophthalmol 2007; 91: 258–262.
Heidenkummer HP, Kampik A, Theirfelder S . Emulsification of silicone oils with specific physiocochemical characteristics. Graefes Arch Clin Exp Ophthalmol 1991; 229: 88–94.
Bartov E, Pennarola F, Savion N, Naveh N, Treister G . A quantitative in-vitro model for silicone oil emulsification. Role of blood constituents. Retina 1992; 12: S23–S27.
Petersen J . The physical and surgical aspects of silicone oil in the vitreous cavity. Graefes Arch Clin Exp Ophthalmol 1987; 225: 452–456.
Dresp JH, Menz D-H . Interaction of different ocular endotamponades as a risk factor for silicone oil emulsification. Retina 2005; 25: 902–910.
Knorr HL, Seltsam A, Holbach L, Naumann GO . Intraocular silicone oil tamponade. A clinico-pathologic study of 36 enucleated eyes. Ophthalmologe 1996; 93: 130–138.
Meinert H . Semifluorinated alkanes and their application. Eur. Patent no. 0003542, 1996.
Tognetto D, Minutola D, Sanguinetti G, Ravalico G . Anatomical and functional outcomes after heavy silicone oil tamponade in vitreoretinal surgery for complicated retinal detachment: a pilot study. Ophthalmology 2005; 112: 1574.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have not received any financial support. The authors have no proprietary or financial interest in any product or device discussed in this paper and have not received any payment as a reviewer or evaluator.
Rights and permissions
About this article
Cite this article
Tognetto, D., Lepori, L., Lapasin, R. et al. A new heavy internal tamponade in vitreoretinal surgery: an in vitro study. Eye 22, 1082–1088 (2008). https://doi.org/10.1038/eye.2008.144
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2008.144