Abstract
To understand the regulation of Helicobacter pylori (H. pylori)-associated gastric carcinogenesis, we examined the effect of B-cell translocation gene 2 (BTG2) expression on the biological activity of Tipα, an oncoprotein secreted from H. pylori. BTG2, the human ortholog of mouse TIS21 (BTG2/TIS21), has been reported to be a primary response gene that is transiently expressed in response to various stimulations. Here, we report that BTG2 is constitutively expressed in the mucous epithelium and parietal cells of the gastric gland in the stomach. Expression was increased in the mucous epithelium following H. pylori infection in contrast to its loss in human gastric adenocarcinoma. Indeed, adenoviral transduction of BTG2/TIS21 significantly inhibited Tipα activity in MKN-1 and MGT-40, human and mouse gastric cancer cells, respectively, thereby downregulating tumor necrosis factor-α (TNFα) expression and Erk1/2 phosphorylation by reducing expression of nucleolin, a Tipα receptor. Chromatin immunoprecipitation proved that BTG2/TIS21 inhibited Sp1 expression and its binding to the promoter of the nucleolin gene. In addition, BTG2/TIS21 expression significantly reduced membrane-localized nucleolin expression in cancer cells, and the loss of BTG2/TIS21 expression induced cytoplasmic nucleolin availability in gastric cancer tissues, as evidenced by immunoblotting and immunohistochemistry. Higher expression of BTG2 and lower expression of nucleolin were accompanied with better overall survival of poorly differentiated gastric cancer patients. This is the first report showing that BTG2/TIS21 inhibits nucleolin expression via Sp1 binding, which might be associated with the inhibition of H. pylori-induced carcinogenesis. We suggest that BTG2/TIS21 is a potential inhibitor of nucleolin in the cytoplasm, leading to inhibition of carcinogenesis after H. pylori infection.
Similar content being viewed by others
Introduction
The proliferation of cancer cells should be supported by their clonal selection to obtain a growth advantage either by oncogene activation or tumor suppressor inactivation. To understand Helicobacter pylori (H. pylori)-associated gastric carcinogenesis, we examined the effect of the expression of B-cell translocation gene 2 (BTG2), the human ortholog of the mouse TIS21 gene (BTG2/TIS21), on the biological activity of Tipα, an oncoprotein secreted from H. pylori. Gastric cancer is the fifth most common malignant cancer in the world and the third leading cause of cancer death.1 The incidence of gastric cancer remains the highest in Japan and Korea with a high prevalence of infection by H. pylori,2 which is a Gram-negative, spiral-shaped bacteria colonized in the human stomach and recognized as a causative agent of chronic gastritis, ulceration and carcinoma based on Hill’s criteria.3 However, cancer development by H. pylori infection has been reported to show a frequency less than 5%, and most cases remain asymptomatic.4 The major antigen of H. pylori identified in 1993 is CagA, a component of cag pathogenicity,5, 6 and several other factors of H. pylori were identified later. Virulence factors of H. pylori, including the cytotoxin-associated gene Pathogenicity Island (cagPAI), CagA, VacA and urease, are well known.7 Thus, we investigate the effect of tumor suppressor gene expression on gastric carcinogenesis caused by the recently identified virulence factor Tipα (TNFα-inducing protein). Tipα is secreted as a homodimer,8 and recombinant Tipα strongly induces TNFα and various chemokines, which can trigger tumor promotion both in vitro and in vivo.9 The secretion of Tipα in gastric cancer is larger than that in gastritis, suggesting that it is a key factor in inflammation related-carcinogenesis. Tipα acts as an epithelial–mesenchymal transition inducer in gastric cancer cells through activating the MEK-ERK pathway.10
Although mouse TIS21 and rat PC3 were initially reported as a primary response gene in 3T3 fibroblasts and pheochromocytoma cells after 12-O-tetradecanoylphorbol-13 acetate and nerve growth factor treatment, respectively,11, 12 BTG2 is constitutively expressed in the epithelial cells of various organs.13 Therefore, the loss of BTG2 expression has been frequently observed in the carcinogenesis of various organs,14, 15, 16, 17, 18, 19, 20, 21, 22 leading to the failure to inhibit cancer progression.21, 22, 23, 24, 25 Loss of BTG2 expression is frequently associated with epigenetic regulation such as DNA methylation at CpG islands,21 histone lysine methylation by SETD1A26 and induction of microRNAs targeting BTG2 expression.22 The characteristic effect of BTG2/TIS21/PC3 gene expression inhibits cell proliferation27 in both normal and cancer cells, and forced expression of BTG2 significantly reduces the cell cycle at the G1/S transition via inhibiting cyclin E/CDK4 and cyclin D biosynthesis, respectively.28, 29 Recent reports have shown that endogenous BTG2 expression is reduced in gastric cancer cells and targeted by microRNAs.30, 31 However, thus far, there is no report regarding the regulation mechanism of H. pylori-associated carcinogenesis by the BTG2/TIS21 gene. BTG2 expression induces various anti-carcinogenic activities, such as G2/M arrest and induction of apoptosis,32, 33, 34 regulation of hematopoietic precursor proliferation, thymocyte expansion, and oxidative damage after doxorubicin treatment.35, 36, 37
It has been known that nucleolin (NCL) is a major non-ribosomal protein in the nucleus of normal cells but was also observed in the surface of cancer and normal cells, and acts as a receptor of various ligands; cell-surface NCL plays a major role in carcinogenesis and various diseases depending on the ligand.38, 39, 40 Cell-surface NCL is critical for Tipα activity because small interfering RNAs against NCL significantly inhibited Tipα-induced epithelial–mesenchymal transition phenotypes.10 NCL is abundant in proliferating cancer cells and much more abundant in gastric cancer than in non-malignant tissues.8, 41
We observed here that BTG2/TIS21 expression was mild in the glandular epithelium of the stomach body before H. pylori infection, whereas it was increased after H. pylori infection compared with the loss of BTG2 expression in adenocarcinoma. This observation strongly suggests a potential role of BTG2/TIS21 in gastric carcinogenesis. Indeed, forced expression of BTG2/TIS21 inhibited Tipα-induced TNFα expression via inhibiting NCL transcription by Sp1. Inhibition of Sp1 activity by BTG2/TIS21 downregulated NCL availability in the membrane of cancer cells. Taken together, BTG2/TIS21 inhibits NCL expression by Sp1 binding, resulting in protection from gastric carcinogenesis associated with H. pylori infection.
Materials and methods
Cell culture
The human gastric cancer cell line MKN-1 was purchased from RIKEN BRC Cell Bank (Ibaraki, Japan) was authenticated by the PowerPlex 16 System (Promega KK, Tokyo, Japan), and was maintained in RPMI 1640 medium (GIBCO, Life Technologies, Grand Island, NY, USA) with 10% fetal bovine serum at 37 °C. The mouse gastric cancer cell line MGT-40 was established from mouse glandular stomach carcinoma. MGT-40 cells were maintained in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum and the MITO+ serum extender (Becton-Dickinson and Company San Diego, CA, USA).
Preparation of recombinant Tipα protein
His-tagged Tipα protein was expressed in Escherichia coli (BL21) and was transformed by the pET28a(+) vector containing Tipα genes, and recombinant Tipα was purified using an Ni2+-loaded Hitrap Chelating column (GE Healthcare Life Sciences, Japan) as described previously.8
Transduction and transfection analyses
Adenovirus-carrying BTG2-HA (Ad-BTG2) or β-galactosidase (Ad-LacZ) produced in our laboratory19 were transduced into MKN-1 and MGT-40 cells for 5 h and then were maintained until 48 h. Exogenous expression was confirmed by immunoblot analysis with the anti-HA antibody or real-time PCR analysis. For small interfering RNA transfection, siControl (50 nM), siBTG2 (targeting BTG2, 50 nM) or siNCL (targeting nucleolin, 50 nM) was transfected into the cells using Lipofectamine 2000. For knockdown experiments, siRNAs were transfected 24 h before adenovirus transduction.
RNA purification and real-time qPCR (RT–qPCR) analysis
For RNA isolation, samples were harvested with 1 ml of TRIzol (Invitrogen, Carlsbad, CA, USA), and 1 μg of the purified RNA was subjected to reverse transcription for cDNA synthesis using Prime-Script reverse transcriptase (Takara, Inc., Kyoto, Japan). To analyze the mRNA expression of target genes, RT–qPCR was performed using the SYBR Green RealHelixTM qPCR kit, (NanoHelix, Daejeon, Republic of Korea) and the primers indicated in Supplementary Table 1. GAPDH was used as an internal control in the present study.
Immunoblot (IB) analysis
Cell lysates were subjected to SDS–PAGE, and resolved proteins were transferred to nitrocellulose membranes. The membranes were incubated with primary antibodies at 4 °C overnight and then with a horseradish peroxidase-conjugated secondary antibody. Protein bands were visualized using a chemiluminescence kit (AbClon, Inc., Seoul, Republic of Korea). The primary antibodies against HA, α-tubulin, caveolin were obtained from Santa Cruz (Dallas, TX, USA), pERK1/2 was obtained from Cell Signaling (Danvers, MA, USA), and TNFα was purchased from Cell Signaling. The polyclonal anti-nucleolin (anti-NUC295) antibody was kindly provided by Dr Kazuo Hirano.
Chromatin immunoprecipitation (ChIP) assay
ChIP was performed as described previously.42 The DNA samples recovered by phenol-chloroform extraction and ethanol precipitation were re-suspended in nuclease-free water for PCR amplification.
Subcellular fractionation
Cell fractionation was performed according to a previously described protocol (http://www.abcam.com/ps/pdf/protocols/subcellular_fractionation.pdf). Lysates of MKN-1 transduced with Ad-LacZ/Ad-BTG2 were centrifuged at 700 g for 10 min, and the pellet was resuspended in RIPA buffer (nuclear fraction). The supernatant was further centrifuged at 100000 g for 1 h, and the supernatant was used as the cytosolic fraction. The pellet was washed again and re-suspended in RIPA buffer (membrane fraction).
Immunohistochemistry (IHC) of human gastric cancer tissues
Human gastric cancer specimens obtained from the Saitama Cardiovascular and Respiratory Center in Japan from April 2013 to January 2017 were formalin fixed and paraffin embedded. None of the patients had received preoperative adjuvant chemotherapy or radiotherapy. The research proposal was approved by the ethical committees of the Saitama Cardiovascular and Respiratory Center and Saitama University in Japan and Ajou University in the Republic of Korea. All of the cancer and surrounding normal tissues (32 samples each) were excised in the operating room after informed consent was obtained from all the patients, and the paraffin blocks were used for this study. IHC staining was performed by incubating with anti-BTG2 (1:200, ab58219, Abcam, Cambridge, UK) or anti-nucleolin (1:100, Nuc4E2, Abcam) antibodies for 40 min at 37 °C, and the secondary antibody (N-Histofine Simple Stain MAX-PO (Multi), Nichirei Bioscience, Inc. Tokyo, Japan) was applied after blocking with Protein Block (DAKO, Tokyo, Japan). The specificity of the anti-BTG2 and anti-nucleolin antibodies were examined by heat denaturation or autoclave treatment (Supplementary Figures 1a and b). The relative staining intensity of BTG2 and nucleolin (NCL) in stomach tissues was reviewed by pathologists in both Japan and Korea independently (Supplementary Figure 1c). Nuclear and cytoplasmic expression of NCL was magnified (Supplementary Figure 1d).
Statistical analysis
The results are expressed as the means±s.d. based on the control. Statistical significance was analyzed by Student’s t-test. A p-value less than 0.05 was considered to be statistically significant.
Results
The expression of BTG2/TIS21 is increased in mucous epithelium infected with H. pylori but is lost in human gastric adenocarcinoma
BTG2/TIS21 expression is frequently lost in various cancers; hence, we analyzed the changes in BTG2 expression in normal glandular epithelium and adenocarcinoma with or without H. pylori infection by IHC. BTG2/TIS21 expression was detected in the neck portion of gastric glands of the body of the human stomach with H. pylori infection at × 200 (Figure 1a), whereas the expression was absent in adenocarcinoma compared with the expression in normal mucous epithelium at × 40 (Figure 1b). The inset shows the high-power view revealing no BTG2 expression in carcinoma cells. The mucous epithelium did not express BTG2 without H. pylori infection (arrows), whereas it was strongly positive in the parietal cells (rectangle) at × 200 (Figure 1c); however, the expression was increased in the glandular epithelium with increased H. pylori infection (arrows) at × 400 (Figure 1d). When IHC was performed with serial sections of the tissues, Tipα expression and H. pylori infection could be observed (Figure 1e); panels (ii) and (iii) show mild to strong expression of Tipα, respectively, in the mucous glands of the stomach with H. pylori (arrows) infection, whereas no Tipα expression was observed in the mucous glands of the stomach in panel (i) without H. pylori infection (arrow heads) at × 400. The antibody specificity for IHC was evaluated by heat denaturation and autoclaving of the antibodies (Supplementary Figures 1a and b).
BTG2/TIS21 expression is increased in normal mucous epithelium with H. pylori infection, but absent in stomach cancer cells. Immunohistochemistry (IHC) findings of BTG2/TIS21 expression on the serial sections of normal mucosa and cancer tissues of the human stomach infected with H. pylori. (a) BTG2 expression was increased in the neck portion of gastric glands in the body of the stomach with H. pylori infection, × 200. (b) BTG2 expression was lost in adenocarcinoma despite the mild expression in normal mucous epithelium, × 40. Inset is the high-power view revealing no BTG2 expression in carcinoma cells. (c) Absence of BTG2 expression in mucous epithelium (arrows) compared with strong positive expression in the parietal cells (rectangle) of the human stomach without H. pylori infection, × 200. (d) Induction of BTG2 expression in the surface of mucous glands infected with many H. pylori (arrows), × 400. (e) To confirm the secretion of Tipα and H. pylori infection, serial sections of paraffin blocks were stained. Note mild (panel ii) to strong (panel iii) expression of Tipα and H. pylori in the mucous glands of the stomach with H. pylori (arrows) infection but no Tipα expression (panel i) in the mucous glands of the stomach without H. pylori infection (arrow heads), × 400.
As expected, BTG2/TIS21 expression was absent in both of the human and mouse gastric cancer cell lines MKN-1 and MGT-40 (Supplementary Figure 2). However, treating the cells with the hypomethylating agent Decitabine significantly increased BTG2/TIS21 expression in the cell lines (Supplementary Figure 3). Furthermore, methylation-specific PCR analysis revealed that the BTG2 gene was indeed methylated, and the methylation can be removed upon Decitabine treatment (Supplementary Figure 3e). To examine the antiproliferative effect of BTG2/TIS21 expression in gastric cancer cells, growth of the MKN-1 and MGT-40 cells transduced with either Ad-LacZ or Ad-BTG2 was measured, and we found significant inhibition of their growth in the BTG2 expresser compared with that in the LacZ control (p<0.001); 40% were reduced in MKN-1 cells, and 50% were decreased in MGT-40 cells (Supplementary Figures 4a and b). To evaluate whether BTG2 is secreted from normal cells, IP and IB analyses were performed with culture media of the MKN-1 cells transduced with adenoviruses. We found the secretion of BTG2 protein only in the BTG2 expressers not the LacZ expresser (Supplementary Figure 4c). The data strongly supported our hypothesis that BTG2 protein released from normal gastric epithelial cells might be active in suppressing the proliferation of glandular epithelial cells, although further studies are required.
Inhibition of Tipα activity by BTG2/TIS21 in human gastric cancer cells
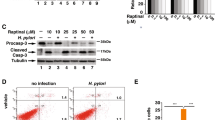
To understand the role of BTG2/TIS21 in Tipα-induced carcinogenesis, TNF-α mRNA expression was examined by RT–qPCR in MKN-1 and MGT-40 cells infected with either Ad-BTG2 or Ad-LacZ virus. TNF-α expression was significantly induced by Tipα (100 μg ml−1) treatment in the both cells with Ad-LacZ infection; however, Tipα-induced TNF-α expression was significantly reduced in cells infected with Ad-BTG2 (upper panels in Figures 2a and b). The second panels showed exogenous BTG2 expression induced by Ad-BTG2 transduction. To confirm the activity of BTG2 in the regulation of Tipα-activity, IB analysis was performed. As shown in the third panel (Figures 2a and b), Tipα-induced TNFα protein expression was significantly reduced by the transduced BTG2 gene in both MKN-1 and MGT-40 cells, suggesting a possible inhibitory role of BTG2 in gastric carcinogenesis. We also examined the role of endogenous BTG2 in the inhibition of Tipα activity by treating cells with the demethylating agent Decitabine; treatment significantly induced endogenous BTG2 expression in a concentration- and time-dependent manner in both MKN-1 and MGT40 cells (Supplementary Figures 3a–d). DNA methylation in the BTG2 gene was efficiently removed by 0.5 μM Decitabine treatment for 4 days (Supplementary Figure 3e) along with blocking Tipα (100 μg ml−1)-induced TNFα transcription (Supplementary Figure 3f). The above data strongly support that endogenous BTG2 expression is also active in the inhibition of Tipα activity in gastric carcinogenesis. In addition, BTG2 expression also inhibited Tipα-induced Erk activity in MKN-1 cells, when it was evaluated by readout of its target phospho-RSK1 (upper panel, Figure 2c). Densitometric analysis revealed that the 1.6-fold increased p-Erk1/2 in the LacZ expresser by Tipα treatment was reduced approximately 30% in the BTG2 expresser (lower panel in Figure 2c). The inhibitory effect of BTG2 on Tipα-induced pErk1/2 could be further confirmed by short interfering RNAs (siBTG2, Figure 2d); knockdown of BTG2 expression maintained Tipα-induced pErk1/2 in MKN-1 cells, and the effect was also confirmed in MGT-40 cells (data not shown). Activation/suppression of Erk1/2 by Tipα/BTG2 might affect the growth rate of gastric cancer cells; BTG2 expression itself significantly inhibited the growth of MKN-1 cells compared with that of the LacZ expressed, and it was independent of Tipα treatment (Supplementary Figure 4d). The above data strongly support the possibility that BTG2/TIS21 inhibits the oncogenic activity of Tipα secreted by H. pylori.
Tipα activity is significantly reduced by BTG2 expression in human gastric cancer cells. To evaluate the effect of BTG2 expression on Tipα activity, Tipα-induced TNFα expression and ERK1/2 activation were examined in the human and mouse gastric cancer cells MKN-1 (a) and MGT-40 (b), respectively, after transduction of the cells with 100 MOI of adenovirus carrying the LacZ or BTG2 gene for 5 h. The cells were maintained until 48 h before treatment with either Tipα (100 μg ml−1) or vehicle for 1 h. Cellular RNAs were isolated to examine the regulation of TNFα expression by RT-qPCR. GAPDH expression was used as an internal control. Immunoblot analysis was also performed using the above samples, TNFα protein expression was analyzed, and α-tubulin served as a loading control. Tipα-induced TNFα expression was significantly reduced in the BTG2 expresser compared with that in the LacZ control. (c) Immunoblot (IB) analysis and quantitation. Tipα-induced ERK1/2 activation was significantly reduced in MKN-1 cells with BTG2 expression compared with that in the LacZ control. pRSK1, a downstream target of pERK1/2, was also reduced. α-Tubulin serves as a loading control (upper panel). Densitometric analysis was performed using ImageJ software (Lower panel); regulation of Tipα-induced pERK1/2 in Ad-LacZ was significantly reduced in the Ad-BTG2-infected cells. (d) Regulation of Tipα-induced ERK1/2 activation by the knockdown of BTG2 expression. To confirm the effect of BTG2 expression on the downregulation of Tipα-induced ERK1/2 activation, MKN-1 cells were transfected with siBTG2 before transduction with Ad-BTG2, and then the cells were subjected to IB analysis. Note the recovery of the BTG2-inhibited pERK1/2 level by siBTG2 transfection.
Reduction of nucleolin expression by BTG2 via inhibiting Sp1 binding to the nucleolin promoter
Based on our report that nucleolin (NCL) serves as a receptor for Tipα entry into gastric cancer cells,40 we evaluated whether BTG2 regulates NCL expression. When RT–qPCR and IB analyses were performed, the mRNA (Figure 3a) and protein (Figure 3b) expression of NCL was significantly reduced in the BTG2 expresser compared with that in the LacZ control. Inhibition of NCL expression by BTG2 gene was independent of Tipα treatment in MKN-1 cells (Figure 3c), and the inhibition was also observed in MGT-40 cells (Supplementary Figure 5). To investigate the mechanism of the inhibition, potential transcription factors bound to the promoter of the NCL gene up to 2 kb were screened using Alibaba 2.2 software (Supplementary Table 2). Based on their frequency, Sp1 binding to the NCL promoter was examined by ChIP, and we observed the strong binding of Sp1 to the NCL promoter, which was well supported by the higher NCL expression in MKN-1 cells than that in normal HDF cells (Supplementary Figure 6). When analyzed by RT–qPCR (Figure 3d) and ChIP (Figure 3e), BTG2 expression significantly inhibited the expression and binding of Sp1 to the NCL promoter. The data were plotted after three independent experiments. When MKN-1 cells were transfected with siBTG2, the BTG2-inhibited Sp1 binding to the NCL promoter was recovered by the ChIP assay with the anti-Sp1 antibody (Figures 3f and g, ***p<0.001). The data were also quantified after three independent experiments. Taken together, BTG2/TIS21 inhibited NCL transcription by downregulating Sp1 binding to the NCL promoter, which might downregulate Tipα-associated gastric carcinogenesis.
Expression of nucleolin, a Tipα receptor, is reduced by BTG2 expression via the inhibition of Sp1 binding to the nucleolin promoter. To investigate whether BTG2 regulates the expression of the Tipα receptor, nucleolin (NCL) mRNA was isolated from MKN-1 cells infected with either Ad-LacZ or Ad-BTG2 before the following analyses: (a) RT-qPCR, in which BTG2 reduced NCL mRNA expression in MKN-1 cells compared with that in LacZ-transduced cells. GAPDH was used as an internal control. (b) IB analysis, in which BTG2 significantly reduced NCL protein expression. α-Tubulin served as a loading control. (c) RT-qPCR analysis, in which NCL expression was lower in the BTG2 expresser than in the LacZ expresser independent of Tipα treatment. (d) RT-qPCR analysis revealed the inhibition of Sp1 expression in MKN-1 cells with BTG2 expression. GAPDH was internal control. (e) Chromatin immunoprecipitation (ChIP) assay. Sp1 binding to the NCL promoter was reduced in the BTG2-transduced MKN-1 cells compared with that in the LacZ-transduced cells. Immunoprecipitation (IP) with normal IgG was employed as the IP control (Left panel). ImageJ analysis was used to analyze Sp1 binding to the NCL promoter in the LacZ- and BTG2-transduced cells. BTG2 transduction significantly downregulated Sp1 binding to the NCL promoter (right panel). (f) IB analysis revealed the knockdown of BTG2 expression by transfection of MKN-1 cells with siBTG2. siControl RNAs were obtained from the scrambled sequences. (g) ChIP assay confirmed the activity of the BTG2 gene in Sp1 binding to the NCL promoter (upper panel). ImageJ analysis was applied, and we found that Sp1 binding was significantly reduced in the BTG2 expresser but was recovered by transfection with siBTG2 (lower panel).
Inverse regulation of Tipα-induced TNFα expression by NCL and BTG2
Because the endogenous expression of NCL was high in the gastric cancer cells (Supplementary Figure 6a) along with the strong binding of Sp1 to its promoter, the regulation of Tipα-induced TNFα expression by BTG2 was reevaluated after the knockdown of NCL expression in MKN-1 cells. Tipα-induced TNFα expression was significantly reduced in the BTG2 expressed; thus, knockdown of BTG2 failed to inhibit TNFα expression (Figure 4a). The data indicate that BTG2 inhibits Tipα-induced TNFα expression independent of the NCL level. Next, when the effect of NCL on Tipα-induced TNFα expression was evaluated, it was significantly reduced in both the LacZ and BTG2 expressers after knockdown of NCL expression (lanes 4 and 8 in Figure 4b) compared with that in the control (lanes 2 and 6 in Figure 4b); however, the level was much higher in the cells with siBTG2 transfection than in the siControl-transfected cells (lanes 8 and 12 in Figure 4b). The data suggest that inverse regulation of Tipα-induced TNFα expression by BTG2 and NCL, as well as the regulation by BTG2 or NCL, was independent of Tipα treatment in MKN-1 cells under the knockdown of BTG2 and NCL genes (Supplementary Figure 7). Immunoblotting analysis was performed to confirm the knockdown efficiency of siRNAs and analyze the expression levels of TNFα, nucleolin and BTG2-HA. α-Tubulin was used as a loading control (Figures 4c, d).
Tipα-induced TNFα expression is inversely regulated by nucleolin and BTG2. To confirm the effect of BTG2 expression on Tipα-induced TNFα expression, endogenous NCL expression was excluded by transfection of MKN-1 cells with siNCL, and then adenoviral transduction was performed. RT-qPCR analysis (a) Tipα-induced TNFα expression was significantly reduced in the BTG2 expresser, but it was recovered by the knockdown of BTG2 with siBTG2 transfection. Expression of GAPDH served as the internal control. (c) IB analysis clearly revealed the regulation of TNFα expression depending on BTG2 expression. Knockdown of nucleolin and BTG2-HA was also revealed. α-tubulin served as an internal control. To explore the effect of NCL on the regulation of Tipα-induced TNFα expression, MKN-1 cells transfected with either siNCL or siBTG2 were transduced with either Ad-LacZ or Ad-BTG2, and then Tipα was treated 1 h before RNA extraction. (b) Note the significant inhibition of Tipα-induced TNFα expression by the knockdown of NCL expression in both the LacZ and BTG2 expressers, indicating the induction of TNFα expression by NCL expression. (d) Immunoblot analysis was performed to analyze the protein expressions of TNFα, nucleolin and BTG2-HA in the present experiment. α-Tubulin served as an internal control. The data strongly suggest that BTG2 and NCL inversely regulate Tipα activity in gastric cancer cells.
Reciprocal expression of BTG2/TIS21 and nucleolin expression in normal and cancer regions
To explore whether BTG2/TIS21 regulates the intracellular localization of NCL protein, cell fractionation was performed in MKN-1 cells, followed by IB analysis. Transduction of Ad-BTG2 into MKN-1 cells clearly reduced membrane-localized NCL expression compared with Ad-LacZ (Figure 5a). When the expression of NCL and BTG2 was examined by IHC in human gastric cancers, BTG2 expression was absent in carcinoma cells (rectangle) compared with the expression in normal tissue (arrow). By contrast, NCL expression was much stronger in the carcinoma (rectangle) than in the normal tissue (Figure 5b), supporting the reciprocal expression of BTG2 and NCL. The expression level was quantified by two pathologists according to the staining score as shown in Supplementary Figure 1c; BTG2 expression in cancer tissue was approximately 1/4 that in normal tissue (26.9±28.3 vs 113.5±61.3, p<0.01), but NCL expression was higher in cancer tissue than in normal tissue (p<0.05, Figure 5c), indicating the loss of BTG2 expression in contrast to the gain of NCL expression in cancer tissue. NCL expression was strong in the nuclei of both normal and cancer tissues, whereas cytoplasmic expression was relatively stronger in the cancer region than in the normal region (Figure 5d, Supplementary Figure 1d). When the frequency was counted under a microscope, that of cytoplasmic NCL was significantly higher in cancer tissue than in normal tissue (15.3±6.6 vs 4.8±4.8, Figure 5e), confirming the reciprocal expression of BTG2/TIS21 and NCL expression in gastric cancer tissue and cell lines.
Gastric cancer expresses nucleolin in the cytoplasm but has lost BTG2 expression. (a) IB analysis. MKN-1 cells transduced with either Ad-LacZ or Ad-BTG2 were fractionated into cytosolic, nuclear and membranous parts and were subjected to IB analysis. NCL expression was downregulated in the membrane fraction of the BTG2 expresser. *indicates NCL proteins detected by IB analysis. α-Tubulin served as a loading control for the cytosol, and caveolin served as the control for the nuclear and membranous fractions. The data represent three independent trials. (b) IHC of human gastric cancer tissues with anti-BTG2 (upper) and anti-NCL (lower) antibodies. BTG2 expression was absent in gastric carcinoma (rectangle) but was still present in normal tissue (arrow). By contrast, NCL expression was strong in the carcinoma (rectangle) compared with that in normal tissue. (c) The extent of BTG2 and NCL expression was scored after staining tumor tissues with anti-BTG2 and anti-NCL antibodies, and then the staining intensity was scored from 0+ to 3+ as shown in Supplementary Figure 1c. BTG2 expression was four times higher in normal than in tumor tissues (p<0.01), whereas NCL expression was higher in tumor than in normal tissues (p<0.05), indicating the loss of BTG2 expression as opposed to the gain of NCL expression in cancer tissues compared with control tissues. (d) IHC with anti-NCL antibody; cytoplasmic expression of NCL was noted in cancer cells but not in normal mucous epithelium. The upper panel shows NCL expression in the nuclei of the gastric gland epithelium in the normal stomach. The lower panel reveals strong expression of NCL in both the cytoplasm and nuclei of cancer cells. (e) NCL expression in the cytoplasm and nuclei was counted under a microscope and was expressed as % of the total cells, *p< 0.05 vs normal. Note the significant increase in the cells with NCL expression out of the nuclei. Supplementary Figure 1d shows representative cells with cytoplasmic NCL expression used for the staining score.
Inverse regulation of overall survival in gastric cancer patients by BTG2/TIS21 and NCL genes
To examine the clinical correlation between the overall survival of gastric cancer and expression of BTG2/TIS21 and NCL, Kaplan–Meier analysis was carried out using the open data. BTG2 high expression significantly increased overall survival compared with the low expressers (p=0.024, Figure 6a), whereas NCL and Sp1 high expression showed a negative impact on the overall survival of the poorly differentiated gastric cancers (p=0.033 in Figures 6b and p=0.055 in Supplementary Figure 8).
Overall survival of gastric cancer is reciprocally regulated by the expression of BTG2/TIS21 and nucleolin. To explore the clinical significance and correlation between the survival of gastric cancer and expression of BTG2 and NCL, we examined the public databases concerning human gastric cancer. The red line indicates the high expresser, and the black one indicates the low expresser. Kaplan–Meier analysis showing the overall survival vs BTG2 and NCL expressions in gastric cancer patients. (a) Note the better survival rate in the poorly differentiated gastric cancer patients with BTG2 high expression than in those with BTG2 low expression (p=0.0024), whereas it was statistically insignificant in the well-differentiated gastric cancers. (b) By contrast, the NCL high expresser exhibited a lower survival rate in the poorly differentiated gastric cancers compared with the low expressers (p=0.033). The correlation was also statistically insignificant in the well-differentiated gastric cancers. The numbers indicate the surviving patients at each time point.
Discussion
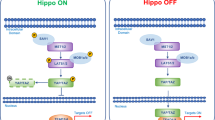
We presented here a potential role of BTG2/TIS21 expression in the inhibition of H. pylori-associated gastric carcinogenesis (Figure 7); Tipα-induced TNFα expression and ERK1/2 activation was downregulated by BTG2/TIS21 expression in gastric cancer cells (Figure 2) through the reduction of NCL expression, a receptor of Tipα, by inhibiting Sp1 binding to the promoter of the NCL gene (Figure 3). BTG2/TIS21 expression is strong in parietal cells of the stomach and mild in normal glandular epithelium without H. pylori infection (Figures 1a and c); however, it was increased with H. pylori infection (Figure 1d). BTG2-negative surface epithelial cells can gain a growth advantage along with clonal selection, leading to the absence of BTG2/TIS21 expression in human gastric adenocarcinoma (Figure 1b). Loss of BTG2/TIS21 expression fails to inhibit TNFα expression in response to Tipα secreted by H. pylori (Figure 7). Indeed, the endogenous expression of BTG2/TIS21 in the gastric cancer cell lines MKN-1 and MGT-40 was extremely low (Supplementary Figure 2) due to DNA methylation (Supplementary Figure 3). Loss of BTG2/TIS21 expression by DNA methylation has been reported in various cancer cells.21, 22, 26 Indeed, forced expression of BTG2 in gastric cancer cells significantly reduced cancer cell growth (Supplementary Figures 4a and b) despite treatment with Tipα protein (Supplementary Figure 4d). As already reported, the expression of BTG2/TIS21 inhibits the invasion of bladder cancer to the muscle layer and breast cancer to adjacent lymph nodes in patients,21, 25 implying the inhibitory activity of BTG2/TIS21 in cancer progression. It is quite possible that BTG2/TIS21 protein secreted from normal glandular epithelium (Supplementary Figure 4c) might be active to inhibit cell growth and the carcinogenic process. Moreover, H. pylori can damage the gastric epithelial layer either by inducing apoptosis43 or inhibiting secretion from parietal cells,44 which might turn off BTG2/TIS21 expression in the dysplastic region of the stomach. Because H. pylori infection epigenetically regulates tumor suppressors and regulates gene expression to promote gastric carcinogenesis,45 it is possible that epigenetic suppression of BTG2/TIS21 occurs at the early stage of H. pylori infection to induce gastric carcinogenesis over the years of infection. To our best knowledge, this is the first report that shows protective activity of BTG2/TIS21 in H. pylori-associated gastric carcinogenesis.
BTG2/TIS21 downregulates Helicobacter pylori-associated gastric carcinogenesis by inhibiting Tipα activity via downregulating nucleolin expression by Sp1. Schema depicting a potential role of BTG2/TIS21 in gastric carcinogenesis. BTG2/TIS21 secreted from gastric gland epithelium inhibits Tipα-induced NFκB activation and its downstream activities; thus TNFα and Sp1 expression is inhibited. Consequently, NCL expression is downregulated due to the inhibition of Sp1 expression and its binding to the NCL promoter by BTG2/TIS21. The carcinogenic process after H. pylori infection induces BTG2/TIS21 expression in the gastric gland epithelium; however, the cells with BTG2 expression face growth arrest as opposed to the selective growth advantage in adenocarcinoma without BTG2 expression. The cells with lost BTG2 expression fail to block Tipα-induced TNFα and Sp1 transcription; thus, Sp1 can enhance NCL transcription. NCL transport to the cytoplasmic fraction can act as the receptor of Tipα secreted by H. Pylori in the stomach.
Although we do not know yet how overexpressed NCL moves from the nuclear to the cytoplasmic fraction in gastric cancer cells (Figure 5), when we investigated the mechanisms of the inhibition by BTG2/TIS21, it was not due to the regulation of IκBα degradation in gastric cancer cells (data not shown) but by the regulation of NCL expression via the inhibition of transcription and interaction of Sp1 with the NCL promoter (Figure 3). Therefore, the expression of BTG2/TIS21 and NCL in gastric cancer cells and tissues were inversely regulated (Figure 4) and independent of Tipα treatment (Supplementary Figure 7). Additionally, the regulation of Tipα activity by BTG2/TIS21 might be explained in two steps; one is the transcriptional repression of TNFα after Tipα stimulation, and the other is the inhibition of Sp1 binding to the NCL promoter. As reported previously, pERK1/2 acts as a downstream signal mediator of Tipα that promotes epithelial–mesenchymal transition in gastric cancer,10 whereas Tipα-induced pERK1/2 was downregulated by BTG2/TIS21 expression (Figure 2); Tipα-induced TNFα expression was downregulated by BTG2 but increased by NCL expression (Figure 4), indicating inverse regulation. Furthermore, BTG2 high expression increased the overall survival of the poorly differentiated gastric cancer patients, whereas it showed an opposite trend in the Sp1 and NCL high expressers (Figure 6 and Supplementary Figure 8). Taken together, we conclude that BTG2/TIS21 and NCL genes inversely regulate gastric carcinogenesis, especially in the poorly differentiated gastric cancers. In addition, spatial expression of BTG2/TIS21 and NCL in human gastric cancer was mutually exclusive (Figure 5b). IHC showed that NCL was expressed in the membrane of floating cancer cells rather than in their nuclei (Figures 5d and e), whereas forced expression of BTG2/TIS21 significantly reduced NCL expression in the membrane fraction of the cells (Figure 5a). The data can be supported by reports that cell surface NCL acts as a receptor for various ligands in the process of carcinogenesis40, 46, 47 and further support the underlying mechanism of the inhibition of Tipα activity by BTG2/TIS21. Although anti-cancer DNA aptamers are designed to target the plasma membrane NCL for a phase II clinical trial of acute myeloid leukemia and renal cell carcinoma,48 we strongly suggest that BTG2/TIS21 could serve as an in vivo therapeutic target downregulating membranous NCL expression and inhibiting gastric carcinogenesis.
In conclusion, inhibition of gastric carcinogenesis by BTG2/TIS21 expression is based on the downregulation of TNFα and Sp1 transcription and reduction of the interaction of Sp1 with the NCL promoter, ultimately inhibiting cell proliferation and protecting against cancer progression. Increased expression of NCL somehow leads to its movement into cytoplasm and membranous parts and functions as the receptor of Tipα secreted by H. pylori in gastric carcinogenesis. Constitutive expression of BTG2/TIS21 in the normal glandular epithelium of the stomach might be increased early in gastric carcinogenesis by H. pylori infection; at the same time, the infection induces epigenetic silencing of the BTG2/TIS21 gene by DNA methylation along with the proliferation of the BTG2-negative clones to obtain a growth advantage. Once again, we strongly suggest the BTG2/TIS21 gene as an in vivo target to combat H. pylori-induced carcinogenesis.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386.
Shin A, Kim J, Park S . Gastric cancer epidemiology in Korea. J Gastric Cancer 2011; 11: 135–140.
Hill AB . The environment and disease: association or causation? Proc R Soc Med 1965; 58: 295–300.
Wroblewski LE, Peek RM Jr, Wilson KT . Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev 2010; 23: 713–739.
Tummuru MK, Cover TL, Blaser MJ . Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun 1993; 61: 1799–1809.
Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA 1993; 90: 5791–5795.
Atherton JC . The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol 2006; 1: 63–96.
Suganuma M, Kurusu M, Suzuki K, Nishizono A, Murakami K, Fujioka T et al. New tumor necrosis factor-alpha-inducing protein released from Helicobacter pylori for gastric cancer progression. J Cancer Res Clin Oncol 2005; 131: 305–313.
Suganuma M, Yamaguchi K, Ono Y, Matsumoto H, Hayashi T, Ogawa T et al. TNF-alpha-inducing protein, a carcinogenic factor secreted from H. pylori, enters gastric cancer cells. Int J Cancer 2008; 123: 117–122.
Watanabe T, Takahashi A, Suzuki K, Kurusu-Kanno M, Yamaguchi K, Fujiki H et al. Epithelial-mesenchymal transition in human gastric cancer cell lines induced by TNF-alpha-inducing protein of Helicobacter pylori. Int J Cancer 2014; 134: 2373–2382.
Fletcher BS, Lim RW, Varnum BC, Kujubu DA, Koski RA, Herschman HR . Structure and expression of TIS21, a primary response gene induced by growth factors and tumor promoters. J Biol Chem 1991; 266: 14511–14518.
Bradbury A, Possenti R, Shooter EM, Tirone F . Molecular cloning of PC3, a putatively secreted protein whose mRNA is induced by nerve growth factor and depolarization. Proc Natl Acad Sci USA 1991; 88: 3353–3357.
Melamed J, Kernizan S, Walden PD . Expression of B-cell translocation gene 2 protein in normal human tissues. Tissue Cell 2002; 34: 28–32.
Lim IK, Lee MS, Lee SH, Kim NK, Jou I, Seo JS et al. Differential expression of TIS21 and TIS1 genes in the various organs of Balb/c mice, thymic carcinoma tissues and human cancer cell lines. J Cancer Res Clin Oncol 1995; 121: 279–284.
Ficazzola MA, Fraiman M, Gitlin J, Woo K, Melamed J, Rubin MA et al. Antiproliferative B cell translocation gene 2 protein is down-regulated post-transcriptionally as an early event in prostate carcinogenesis. Carcinogenesis 2001; 22: 1271–1279.
Struckmann K, Schraml P, Simon R, Elmenhorst K, Mirlacher M, Kononen J et al. Impaired expression of the cell cycle regulator BTG2 is common in clear cell renal cell carcinoma. Cancer Res 2004; 64: 1632–1638.
Kawakubo H, Carey JL, Brachtel E, Gupta V, Green JE, Walden PD et al. Expression of the NF-kappaB-responsive gene BTG2 is aberrantly regulated in breast cancer. Oncogene 2004; 23: 8310–8319.
Farioli-Vecchioli S, Tanori M, Micheli L, Mancuso M, Leonardi L, Saran A et al. Inhibition of medulloblastoma tumorigenesis by the antiproliferative and pro-differentiative gene PC3. FASEB J 2007; 21: 2215–2225.
Park TJ, Kim JY, Oh SP, Kang SY, Kim BW, Wang HJ et al. TIS21 negatively regulates hepatocarcinogenesis by disruption of cyclin B1-Forkhead box M1 regulation loop. Hepatology 2008; 47: 1533–1543.
Liu M, Wu H, Liu T, Li Y, Wang F, Wan H et al. Regulation of the cell cycle gene, BTG2, by miR-21 in human laryngeal carcinoma. Cell Res 2009; 19: 828–837.
Devanand P, Kim SI, Choi YW, Sheen SS, Yim H, Ryu MS et al. Inhibition of bladder cancer invasion by Sp1-mediated BTG2 expression via inhibition of DNA methyltransferase 1. FEBS J 2014; 281: 5581–5601.
Frampton AE, Castellano L, Colombo T, Giovannetti E, Krell J, Jacob J et al. Integrated molecular analysis to investigate the role of microRNAs in pancreatic tumour growth and progression. Lancet 2015; 385 (Suppl 1): S37.
Lim SK, Choi YW, Lim IK, Park TJ . BTG2 suppresses cancer cell migration through inhibition of Src-FAK signaling by downregulation of reactive oxygen species generation in mitochondria. Clin Exp Metastasis 2012; 29: 901–913.
Choi JA, Lim IK . TIS21/BTG2 inhibits invadopodia formation by downregulating reactive oxygen species level in MDA-MB-231 cells. J Cancer Res Clin Oncol 2013; 139: 1657–1665.
Choi JA, Jung YS, Kim JY, Kim HM, Lim IK . Inhibition of breast cancer invasion by TIS21/BTG2/Pc3-Akt1-Sp1-Nox4 pathway targeting actin nucleators, mDia genes. Oncogene 2016; 35: 83–93.
Tajima K, Yae T, Javaid S, Tam O, Comaills V, Morris R et al. SETD1A modulates cell cycle progression through a miRNA network that regulates p53 target genes. Nat Commun 2015; 6: 8257.
Matsuda S, Rouault J, Magaud J, Berthet C . In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Lett 2001; 497: 67–72.
Lim IK, Lee MS, Ryu MS, Park TJ, Fujiki H, Eguchi H et al. Induction of growth inhibition of 293 cells by downregulation of the cyclin E and cyclin-dependent kinase 4 proteins due to overexpression of TIS21. Mol Carcinog 1998; 23: 25–35.
Guardavaccaro D, Corrente G, Covone F, Micheli L, D'Agnano I, Starace G et al. Arrest of G(1)-S progression by the p53-inducible gene PC3 is Rb dependent and relies on the inhibition of cyclin D1 transcription. Mol Cell Biol 2000; 20: 1797–1815.
Zhang L, Huang H, Wu K, Wang M, Wu B . Impact of BTG2 expression on proliferation and invasion of gastric cancer cells in vitro. Mol Biol Rep 2010; 37: 2579–2586.
Zhou L, Liang X, Zhang L, Yang L, Nagao N, Wu H et al. MiR-27a-3p functions as an oncogene in gastric cancer by targeting BTG2. Oncotarget 2016; 7: 51943–51954.
Ryu MS, Lee MS, Hong JW, Hahn TR, Moon E, Lim IK . TIS21/BTG2/PC3 is expressed through PKC-delta pathway and inhibits binding of cyclin B1-Cdc2 and its activity, independent of p53 expression. Exp Cell Res 2004; 299: 159–170.
Hong JW, Ryu MS, Lim IK . Phosphorylation of serine 147 of tis21/BTG2/pc3 by p-Erk1/2 induces Pin-1 binding in cytoplasm and cell death. J Biol Chem 2005; 280: 21256–21263.
Choi OR, Ryu MS, Lim IK . Shifting p53-induced senescence to cell death by TIS21(/BTG2/Pc3) gene through posttranslational modification of p53 protein. Cell Signal 2016; 28: 1172–1185.
Kim BC, Ryu MS, Oh SP, Lim IK . TIS21/(BTG2) negatively regulates estradiol-stimulated expansion of hematopoietic stem cells by derepressing Akt phosphorylation and inhibiting mTOR signal transduction. Stem Cells 2008; 26: 2339–2348.
Konrad MA, Zuniga-Pflucker JC . The BTG/TOB family protein TIS21 regulates stage-specific proliferation of developing thymocytes. Eur J Immunol 2005; 35: 3030–3042.
Lim YB, Park TJ, Lim IK . B cell translocation gene 2 enhances susceptibility of HeLa cells to doxorubicin-induced oxidative damage. J Biol Chem 2008; 283: 33110–33118.
Storck S, Shukla M, Dimitrov S, Bouvet P . Functions of the histone chaperone nucleolin in diseases. Subcell Biochem 2007; 41: 125–144.
Ginisty H, Sicard H, Roger B, Bouvet P . Structure and functions of nucleolin. J Cell Sci 1999; 112 (Pt 6): 761–772.
Watanabe T, Tsuge H, Imagawa T, Kise D, Hirano K, Beppu M et al. Nucleolin as cell surface receptor for tumor necrosis factor-alpha inducing protein: a carcinogenic factor of Helicobacter pylori. J Cancer Res Clin Oncol 2010; 136: 911–921.
Qiu W, Zhou F, Zhang Q, Sun X, Shi X, Liang Y et al. Overexpression of nucleolin and different expression sites both related to the prognosis of gastric cancer. APMIS 2013; 121: 919–925.
Sundaramoorthy S, Ryu MS, Lim IK . B-cell translocation gene 2 mediates crosstalk between PI3K/Akt1 and NFkappaB pathways which enhances transcription of MnSOD by accelerating IkappaBalpha degradation in normal and cancer cells. Cell Commun Signal 2013; 11: 69.
Xia HH, Talley NJ . Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis. Am J Gastroenterol 2001; 96: 16–26.
Beales IL, Calam J . Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut 1998; 42: 227–234.
Nardone G, Compare D, De Colibus P, de Nucci G, Rocco A . Helicobacter pylori and epigenetic mechanisms underlying gastric carcinogenesis. Dig Dis 2007; 25: 225–229.
Hirano K, Miki Y, Hirai Y, Sato R, Itoh T, Hayashi A et al. A multifunctional shuttling protein nucleolin is a macrophage receptor for apoptotic cells. J Biol Chem 2005; 280: 39284–39293.
Suganuma M, Watanabe T, Yamaguchi K, Takahashi A, Fujiki H . Human gastric cancer development with TNF-alpha-inducing protein secreted from Helicobacter pylori. Cancer Lett 2012; 322: 133–138.
Soundararajan S, Wang L, Sridharan V, Chen W, Courtenay-Luck N, Jones D et al. Plasma membrane nucleolin is a receptor for the anticancer aptamer AS1411 in MV4-11 leukemia cells. Mol Pharmacol 2009; 76: 984–991.
Acknowledgements
This work was supported by grants from the National Research Foundation (No. 2016R1A2B4006466; IK Lim) of the Korean Government MSIP and the International Research & Development Program funded by the Ministry of Education, Science and Technology (MEST) of Korea (NRF-2017K2A9A2A08000213), the Princess Takamastu Cancer Research Fund (13-24511; M Suganuma), Japan Society for the Promotion of Science, Smoking Research Foundation, Takeda Science Foundation, and the Bilateral Joint Program between Korea and Japan. We thank Dr. Kazuo Hirano at Tokyo University of Pharmacy and Life Science for the anti-nucleolin antibody (anti-Nuc295) and Kaori Suzuki, Miki Kanno-Kurusu, and Ikuko Shiotani at Saitama University for their technical assistance.
Compliance with ethical standards: The research proposal was approved by the ethical committees of the Saitama Cardiovascular and Respiratory Center and Saitama University in Japan and Ajou University Hospital in the Republic of Korea. All of the cancer and surrounding normal tissues were excised in the operating room after informed consent was obtained from all of the patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note:
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Devanand, P., Oya, Y., Sundaramoorthy, S. et al. Inhibition of TNFα-interacting protein α (Tipα)-associated gastric carcinogenesis by BTG2/TIS21 via downregulating cytoplasmic nucleolin expression. Exp Mol Med 50, e449 (2018). https://doi.org/10.1038/emm.2017.281
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2017.281
This article is cited by
-
Deregulation of protein phosphatase 2A inhibitor SET is associated with malignant progression in breast cancer
Scientific Reports (2021)
-
TIS21/BTG2 inhibits breast cancer growth and progression by differential regulation of mTORc1 and mTORc2–AKT1–NFAT1–PHLPP2 signaling axis
Journal of Cancer Research and Clinical Oncology (2018)