Abstract
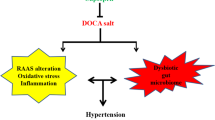
Chronic high-salt diet-associated renal injury is a key risk factor for the development of hypertension. However, the mechanism by which salt triggers kidney damage is poorly understood. Our study investigated how high salt (HS) intake triggers early renal injury by considering the ‘gut-kidney axis’. We fed mice 2% NaCl in drinking water continuously for 8 weeks to induce early renal injury. We found that the ‘quantitative’ and ‘qualitative’ levels of the intestinal microflora were significantly altered after chronic HS feeding, which indicated the occurrence of enteric dysbiosis. In addition, intestinal immunological gene expression was impaired in mice with HS intake. Gut permeability elevation and enteric bacterial translocation into the kidney were detected after chronic HS feeding. Gut bacteria depletion by non-absorbable antibiotic administration restored HS loading-induced gut leakiness, renal injury and systolic blood pressure elevation. The fecal microbiota from mice fed chronic HS could independently cause gut leakiness and renal injury. Our current work provides a novel insight into the mechanism of HS-induced renal injury by investigating the role of the intestine with enteric bacteria and gut permeability and clearly illustrates that chronic HS loading elicited renal injury and dysfunction that was dependent on the intestine.
Similar content being viewed by others
Introduction
The prevalence of hypertension is continuously increasing and is becoming a major public health problem around the world.1, 2 The pathogenesis of hypertension is not fully understood. Renal injury and dysfunction may be one of the main inducers of blood pressure elevation.3, 4 In addition, diets that are high in salt are a direct pathogenic factor for hypertension development, as found by numerous clinical and experimental studies.5 Some evidence indicates that salt uptake is able to upregulate cytokine expression and propagate renal damage,6, 7 however, the relationship between high salt (HS) intake and renal injury development is unclear.
The first organ exposed to dietary salt is the gut. Intestinal abnormalities, including enteric dysbiosis and increased gut permeability, are associated with many extraintestinal diseases. For example, alcohol consumption can induce enteric dysbiosis and can disrupt gut barrier integrity, which allows pathogen-associated molecular patterns to penetrate the blood and translocate into the liver to result in hepatic steatosis and further alcoholic hepatitis.8, 9, 10 This theory prompted us to investigate whether salt uptake is able to directly disrupt intestinal homeostasis and, in turn, trigger early renal injury. To test this hypothesis, we fed mice with HS water for 8 weeks and studied the resulting intestinal pathophysiological changes and their contributions to early renal abnormalities.
Materials and methods
Animals
Six- to eight-week-old male-specific pathogen-free C57BL/6 mice were used. For chronic HS feeding, 2% (w/w) sodium chloride (NaCl) was added to the drinking water for 8 weeks. For antibiotic experiments, polymyxin B (150 mg l−1) and neomycin (200 mg l−1) were added to the NaCl drinking water continuously for 8 weeks. After 8 weeks of feeding, mice were anesthetized and euthanized. Blood, kidney, spleen and liver were harvested in a sterile manner. For blood pressure measurements, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured via the tail cuff method using a non-invasive blood pressure instrument (Softron Biotechnology, Beijing, China). All the measurements were performed between 0900 and 1200 hours. At least six stable results were obtained for each mouse, and the average value was calculated. All mice had free access to food and water and were maintained in a temperature-controlled colony room on a 12:12-h light/dark cycle. Three separate sets of experiments were repeated in this study. All experimental procedures were in compliance with the National Institutes of Health guidelines and were approved by the local Animal Care and Use Committee of the Southern Medical University.
Microbe analysis
Fecal matter or tissue was resuspended in PBS that contained 0.5% Tween 20 and further subjected to a −80 °C/60 °C cycle three times to destroy the membrane. DNA extraction and cecal total bacteria loads were further analyzed as described.11 Mouse intestinal lumen, mucus layer and epithelial layer bacteria isolation was performed as described previously.12 After isolation, total DNA was extracted, diluted to 10 ng μl−1 and quantitative real-time PCR was performed using 16s rRNA primers: 5′-GTGSTGCAYGGYTGTCGTCA-3′; 5′-ACGTCRTCCMCACCTTCCTC-3′; Firmicutes primers: 5′-GGAGYATGTGGTTTAATTCGA-3′; 5′-AGCTGACGACAACCATGCAC-3′; Bacteroidetes primers: 5′-GGCGACCGGCGCACGGG-3′; 5′-GRCCTTCCTCTCAGAACCC-3′. For lumen bacterial quantification, the quantitative PCR value of the 16S rRNA was normalized to weight, and the quantitative PCR value of the 16S rRNA of the mucus and epithelial layer was normalized to the area of the intestine. Renal, hepatic and spleen total DNA was extracted, and 16S rRNA abundance was normalized to host 18S.
A microbial diversity analysis was performed.13 Briefly, the 16S rRNA gene V4 region was amplified and sequenced using the Ion Torrent sequencing platform. The raw sequences were first quality-controlled using QIIME (1.9.1) with default parameters, then demultiplexed and clustered into species-level (97% similarity) operational taxonomic units. Operational taxonomic unit generation is based on GreenGene's database (v13_8) and the reference-based method with SortMeRNA. Strain composition analysis, alpha diversity analysis and beta diversity analysis were also performed using QIIME. Discriminative taxa were determined using LEfSe (LDA Effect Size, http://huttenhower.sph.harvard.edu/galaxy/).
Histological procedures
Tissue was collected and fixed in 10% buffered formalin. The sample was then embedded in paraffin and sliced into 4-μm-thick sections. Hematoxylin and eosin (HE) staining was performed and analyzed by microscopy. Immunofluorescence and immunohistochemistry were performed using the primary antibodies for ki67 (Cell Signaling Technology, Shanghai, China) and IFN-γ (Proteintech, Wuhan, China). A TUNEL kit (KeyGene, Nanjing, China) was used and the staining was performed according to the manufacturer’s instructions. For apoptotic cell quantification, 6–8 random fields per slide were collected, and the positive cells were analyzed. The result of IFN-γ immunohistochemistry was estimated via a semiquantitative scoring system as described previously.14
Gene expression analysis
Total RNA was extracted with Trizol reagent according to the manufacturer’s instructions. A reverse transcript reaction was carried out with a reverse transcript enzyme (TOYOBO, Shanghai, China) according to the manufacturer’s instructions. The real-time PCR reaction was carried out on an ABI 7500 real-time PCR system using primer sequences obtained from NIH qPrimerDepot. A transcriptome analysis was performed as described.15, 16 Briefly, the total RNA was used for Illumina Digital Gene Expression tag profiling processed by BioMarker Technologies (Beijing, China). Library construction was performed according to Illumina instructions and sequenced on an Illumina HiSeq 2500 sequencer (San Diego, CA, USA). The clean tags that mapped to reference sequences from multiple genes were filtered. The remaining clean tags were designed as perfect clean tags. The number of perfect clean tags for each gene was calculated and then normalized in reads per kilobase of exon model per million mapped reads (RPKM) using the previously described method.17 Finally, all of the related genes (referred to KEGG pathways) with RPKM>0 were chosen and compared by log2 (RPKM).
Protein expression and biochemical analysis
Tissue protein was extracted with a commercial lysis buffer (KeyGene). Western blot was performed with the primary antibodies for Occludin, ZO-1, Claudin-2 (Invitrogen, Shanghai, China) and Actin (Proteintech). Fecal and urine albumin were determined via ELISA (Bethyl Labs, Montgomery, AL, USA). The endotoxin level was measured with a commercial kit (GenScript, Nanjing, China). Urine creatinine, Na+ and urea concentration were measured on an automatic biomedical analyzer (Roche, Shanghai, China). Plasma creatinine was determined manually with a commercial kit (BioAssay, Hayward, CA, USA) following the manufacturer’s instructions.
FD-4 permeability experiment
FITC Dextran 4-KD (FD-4, Sigma, Shanghai, China) was used to confirm gut leakiness in vivo. FD-4 was dissolved in PBS and was orally administered to mice at a dose of 0.6 mg kg−1 via a gastric gavage tube. Four hours later, plasma was harvested, and the FD-4 level was measured spectrofluorometrically with an excitation wavelength of 485 nm and an emission wavelength of 530 nm in a microplate fluorescence reader.
Fecal microbiota transplantation (FMT)
Fecal microbiota transplantation was performed according to the modified method described previously.18 Briefly, 6–8-week-old male C57BL/6 mice received antibiotics (vancomycin, 100 mg kg−1; neomycin sulfate 200 mg kg−1; metronidazole 200 mg kg−1; and ampicillin 200 mg kg−1) intragastrically once each day for 1 week to deplete the gut microbiota. The feces of the donor mice (control and chronic HS-fed mice) were collected and resuspended in PBS at 0.125 g ml−1. An amount of 0.15 ml of the solution was administered to mice in the corresponding groups orally via gastric gavage tube once daily in the first week, every other day in the second week, and twice each week from the 3rd week. All the mice were maintained on a regular water and diet, and the feces and urine were collected every week for further examination. Mice were killed 8 weeks after transplantation, and the tissues were harvested for further analysis.
Statistical analysis
Results are expressed as the mean±s.e.m. Statistical evaluations were performed using a two-tailed unpaired Student’s t-test. A P-value of<0.05 was considered a statistically significant difference.
Results
Chronic high salt intake led to enteric dysbiosis
First, we examined whether HS intake could influence intestinal bacteria. Total bacterial load in the cecum slightly decreased without significance after chronic HS feeding (Figure 1a). We further isolated bacteria located in the lumen, mucus layer and epithelial cells from the jejunum, ileum and colon. The bacterial load in the epithelium in the ileum (Figure 1b) and the lumen in the colon (Figure 1b) were also decreased after chronic HS feeding. Next, we analyzed the bacterial composition in the control group of mice compared with the HS-fed mice. Firmicutes and Bacteroidetes are the two main phyla in the gut.19 Figure 2a shows that chronic HS treatment markedly lowered levels of Firmicutes and increased levels of Bacteroidetes, which indicated that the bacterial composition was altered after HS intake. We also performed 16S rRNA sequencing to explore how HS intake influenced the intestinal microflora. The bacterial composition in the HS group was significantly different from that of the control mice in terms of both phylum and class level (as well as other levels, data not shown) in the cecal content (Supplementary Figure 1). In particular, the percentages of Actinobacteria, Firmicutes and Bacteroidetes at the phylum level and Actinobacteria, and Clostridia and Bacteroidia at the class level were markedly altered in the cecum after HS treatment. A principal coordinate analysis plot using the unweighted uniFrac analyses showed that the HS and control groups clustered separately (P=0.002, ADONIS analysis) (Figure 2b). Moreover, according to the linear discriminant analysis along with the effect size measurement, we found that some strains were specifically enriched in one group or the other. For example, Clostridia and Lachnospiraceae were enriched in the control mice, while Burkholderiales and Alcaligenaceae were enriched in the HS-fed mice (Supplementary Figure 2). Other than cecal content, mucosal bacteria are also important factors that influence the gut pathophysiological status.20 The ileal and colonic mucosa had the most bacteria throughout the GI tract. Therefore, we further analyzed the bacteria located in the ileal and colonic mucus layer. The unweighted uniFrac analyses showed that the HS and control groups clustered separately (P=0.014, ADONIS analysis) in the ileal mucus layer (Figure 2c), while the control and HS groups formed two clusters with a clear separation trend (P=0.05, ADONIS analysis) in the colonic mucus layer (Figure 2d). Taken together, our results demonstrated that chronic HS feeding could result in enteric dysbiosis, as evidenced by the changes in bacterial count and microflora composition.
Chronic high salt feeding reduced the bacterial load in the intestine. C57BL/6 mice were fed normal or high salt drinking water for 8 weeks (n=6–9). (a) Total bacterial load in the cecum; (b) bacterial load in the lumen, mucus layer and epithelium in the jejunum, ileum and colon. HS, high salt. The results are expressed as the mean±s.e.m. *P<0.05.
Chronic high salt feeding caused enteric dysbiosis. C57BL/6 mice were fed normal or high salt drinking water for 8 weeks (n=6–10). (a) Relative amounts of Firmicutes and Bacteroidetes in the cecum; (b–d) scatter plot of 3D PCoA for the microbiota in the cecal content, ileal mucus layer and colonic mucus layer. HS, high salt. The results are expressed as the mean±s.e.m. *P<0.05. 3D, three-dimensional; PCoA, principal coordinate analysis.
Chronic high salt feeding caused gut abnormalities characterized by impaired inflammatory responses
Enteric dysbiosis is normally associated with a change in gut pathophysiology.21, 22 We next examined the effect of chronic HS feeding on intestinal changes. There was no difference in the morphology of the epithelium in both the ileum and the colon between the control and the HS-fed mice (Figure 3a). Moreover, similar numbers of apoptotic and proliferated cells, identified via TUNEL and ki67 staining, indicated that chronic HS feeding did not influence cell death in the intestine (Figure 3a). However, we found that inflammatory marker expression was dramatically altered by chronic HS intake. In particular, Ccl4, Ccl5 and Ifn-γ displayed higher mRNA levels in the ileum in HS mice (Figure 3b). The results of IFN-γ immunohistochemistry in the ileum confirmed the gene expression data (Figure 3c). As TLR family proteins and Nf-κB are the main molecules involved in the pathogen-associated immunological response, we further evaluated TLR family and Nf-κB gene expression and found that TLR2, TLR3 and TLR5 mRNA levels trended higher in the ileum (Figure 3d). Meanwhile, the expressions of IRF7, GATA3 and NFKB2 were significantly upregulated after HS treatment (Figure 3e). Moreover, the expression of CD38 in white blood cells was elevated in the ileum after HS feeding (Figure 3f). We then performed a transcriptome analysis via RNA sequencing and compared all of the related genes involved in ‘cytokine–cytokine receptor interaction’, ‘NF-κB signaling pathway’ and ‘Toll-like receptor signaling pathway’ between the control and the HS groups. In both the ileum and the colon, the expression of many genes was different between the two groups (Supplementary Figure 3). Collectively, these data indicated that chronic HS intake caused intestinal inflammatory response disruption.
Chronic high salt feeding was associated with gut inflammatory response disruption. C57BL/6 mice were fed normal or high salt drinking water for 8 weeks (n=6–12). (a) HE, TUNEL and ki67 staining; (b) mRNA levels of key cytokines and chemokines in the ileum; (c) IFN-γ immunohistochemistry in the ileum and the quantification analysis (n=3); (d) mRNA levels of TLRs in the ileum; (e) mRNA levels of genes involved in the Nf-κB signaling pathway in the ileum; (f) CD expression in the ileum. Scale bar, 100 μm. HS, high salt. The results are expressed as the mean±s.e.m. *P<0.05.
Chronic high salt intake resulted in the loss of intestinal barrier function and promoted bacterial translocation into the kidney
Impaired immunological responses in the intestine are always associated with gut barrier dysfunction.23 We then investigated how HS intake affected gut barrier function. Intestinal permeability was monitored by fecal albumin content, which showed that HS feeding promoted gut leakiness (Figure 4a). Meanwhile, HS intake markedly decreased tjp-1, tjp-2, claudin-1, claudin-7 and claudin-8 gene expression in the colon (Figure 4b). In addition, barrier-forming tight-junction ZO-1 protein levels in the ileum and the colon, and Occludin protein levels in the colon, displayed markedly lower trends after HS intake compared with the control mice (Figures 4c and d). On the other hand, pore-forming tight junction Claudin-2 protein levels were significantly higher after HS feeding in both the ileum and the colon (Figures 4c and d). These data clearly demonstrated that the intestinal gut barrier was disrupted by chronic HS intake.
Chronic high salt feeding led to gut barrier dysfunction and promoted bacterial translocation into the kidney. C57BL/6 mice were fed normal or high salt drinking water for 8 weeks (n=6–7). (a) Fecal albumin content; (b) tight junction mRNA levels in the colon; (c) ZO-1 and Claudin-2 protein levels in the ileum; (d) occludin, ZO-1 and Claudin-2 protein levels in the colon; (e) bacterial translocation indicated by the relative 16S/18S ratio in the kidney, liver and spleen; (f) LDA along with effect size measurements was applied to show the enriched bacteria in the high salt kidney (n=3). HS, high salt. The results are expressed as the mean±s.e.m. *P<0.05. LDA, linear discriminant analysis.
Increased gut permeability could lead to gut bacteria or bacterial product translocation into extraintestinal tissue. By using 16s PCR to recognize bacterial DNA, we detected bacterial translocation in the kidney, liver and spleen. Interestingly, chronic HS intake specifically promoted bacterial translocation into the kidney but not into the liver or the spleen (Figure 4e). We further analyzed the renal bacteria and found that Bacillales, which belongs to enteric bacteria,24 was found to be enriched in the HS-treated kidney compared with controls based on the LEfSe measurement (Figure 4f). In particular, the levels of Bacillus and Planomicrobium were increased 2.6-fold and 8.7-fold, respectively, in the HS-fed kidneys compared with the kidneys in the control animals. These results indicated that chronic HS intake could promote the translocation of certain bacteria. Our data clearly demonstrated that HS feeding is associated with gut barrier disruption and gut bacteria translocation into the kidney.
Chronic high salt feeding associated intestinal barrier disruption and renal injury depend on gut microbiota
To further clarify the association between enteric dysbiosis and renal injury after HS intake, we fed mice polymyxin B and neomycin, which have been described as non-absorbable antibiotics that are able to deplete gut bacteria.25, 26 Antibiotic administration did restore HS feeding-induced ileal Ifn-γ overexpression (Figure 5a), gut leakiness as monitored by fecal albumin and FD-4 penetration (Figures 5b and c), and bacterial translocation as monitored by both the plasma endotoxin level and the kidney 16s/18s ratio (Figures 5d and e). Antibiotics did not alter sodium loading as measured by urine and plasma Na+ concentration (Figure 6a). Although our 8-week HS feeding did not cause advanced renal damage such as fibrosis, as monitored by col1a1 mRNA levels (Figure 6b), HS feeding increased plasma creatinine levels, and antibiotic treatment was associated with a lower, though not significant, trend of plasma creatinine levels compared with HS treatment (Figure 6c). More importantly, the urine albumin/creatinine ratio that was the main marker for renal dysfunction was significantly increased in HS-fed mice when compared with control mice, and antibiotic treatment could almost completely restore renal function (Figure 6d). In addition, TUNEL staining revealed that antibiotic administration could reduce HS-induced apoptotic cell elevation (Figures 6e and f). In conclusion, our data showed that antibiotic treatment is able to ameliorate gut leakiness and early renal injury caused by HS feeding.
Antibiotics restored gut leakiness induced by chronic high salt feeding. C57BL/6 mice were fed normal water, high salt drinking water and antibiotics (polymyxin B and neomycin) contained in high salt drinking water for 8 weeks (n=6–15). (a) Ileal Ifn-γ mRNA levels; (b) fecal albumin content; (c) FITC-Dextran (4 kDa, FD-4) level in the plasma; (d) relative plasma endotoxin level; (e) relative 16S/18S ratio in the kidney. ABX, antibiotics; HS, high salt. The results are expressed as the mean±s.e.m. *P<0.05.
Antibiotics ameliorated early renal injury induced by chronic high salt feeding. C57BL/6 mice were fed normal water, high salt drinking water and antibiotics (polymyxin B and neomycin) contained in high salt drinking water for 8 weeks (n=6–8). (a) Na+ concentration in the urine and plasma; (b) renal col1a1 mRNA level; (c) plasma creatinine level; (d) urine albumin/creatinine ratio; (e, f) TUNEL staining in the kidney and the quantification analysis. Scale bar, 100 μm. ABX, antibiotics; HS, high salt. The results are expressed as the mean±s.e.m. *P<0.05.
Systolic blood pressure elevation induced by chronic high salt feeding depends on gut microbiota
Chronic HS feeding may lead to arterial pressure elevation, and we also detected how antibiotics affected this progression. As presented in Figure 7, although DBP and mean blood pressure were not increased in HS-treated mice, SBP was significantly elevated after chronic HS feeding. Interestingly, antibiotic treatment was able to completely restore the elevation. These data strongly demonstrated that the elevation in SBP induced by chronic HS feeding depends on the gut microbiota.
Antibiotics restored the blood pressure elevation induced by chronic high salt feeding. C57BL/6 mice were fed normal water, high salt drinking water and antibiotics (polymyxin B and neomycin) contained in high salt drinking water for 8 weeks (n=8–9). (a) The SBP; (b) MBP; and (c) DBP in each group. ABX, antibiotics; HS, high salt; MBP, mean blood pressure. The results are expressed as the mean±s.e.m. *P<0.05.
The gut microbiota from chronic high-salt-treated mice could independently cause gut leakiness and early renal injury
Finally, to further demonstrate that the gut microbiota was the upstream factor for renal injury development, we performed a fecal microbiota transplantation experiment. Two groups of mice that were fed a normal diet (not high salt) were orally administered with feces from control and HS-treated mice. Although the sodium concentration in the cecal content showed an increased trend in HS-fed mice compared with that in control mice (Supplementary Figure 4), the absolute level of sodium that was transferred with the feces was quite small, and the effects of redundant sodium could be ignored. In addition, 8 weeks after transplantation, the cecal Firmicutes/Bacteroidetes ratio, which is the main marker of microbiota composition alteration, was significantly higher in the control mice that received control feces than in the HS mice that received HS feces, which was in agreement with our previous data that showed that HS feeding reduced the Firmicutes/Bacteroidetes ratio in the cecum and demonstrated that our fecal microbiota transplantation experiment was successful (Figure 8a). Furthermore, as presented in Figures 8b and c, 4 weeks after administration, gut permeability, as monitored by fecal albumin content, was significantly increased in the mice that received HS-fed feces, and, importantly, the urine albumin/creatinine ratio, the main phenotype of the renal dysfunction observed in the current study, was also significantly increased in the mice that received HS-fed feces 4 weeks after transplantation. Finally, the numbers of renal apoptotic cells were significantly increased in HS feces transplanted mice compared with control mice (Figures 8d and e). These data further indicated that the enteric dysbiosis caused by HS feeding is the original trigger for renal injury and dysfunction.
Gut leakiness and renal injury were transferrable via gut microbiota. C57BL/6 mice were intragastrically administered with feces from control mice and chronic high-salt-treated mice for the indicated weeks (n=5–9). (a) The Firmicutes/Bacteroidetes ratio in the cecal content 8 weeks after transplantation. (b) Relative fecal albumin content and (c) relative urine albumin/creatinine ratio. (d, e) TUNEL staining in the kidney and the quantification analysis 8 weeks after transplantation. Scale bar, 100 μm. HS, high salt. The results are expressed as the mean±s.e.m. *P<0.05.
Discussion
Although it is well established that an HS diet is able to induce renal injury, the original trigger that induces early injury is not fully known. In our study with mice, 8 weeks of chronic HS feeding resulted in an increased number of renal apoptotic cells and increased renal function impairment; such early renal damage was attributed to enteric bacteria translocation into the kidney. The translocation occurred due to gut barrier loss, which resulted from HS-induced dysbiosis. Our findings provided a novel insight into the ‘gut-kidney axis’. To our knowledge, current ‘gut-kidney axis’ data mainly pertain to the effects of nutrition or hormones derived from the intestine with other neural pathways in the kidneys.27, 28 There is no evidence to show that pathogen-associated molecular patterns derived from the intestine could directly damage the kidney. As such, our study is a novel addition to the ‘gut-kidney axis’ theory and uncovers a novel fundamental pathogenesis for HS-linked early renal damage development.
Salt is mainly absorbed in the intestines, as are other oral substances in the diet, such as alcohol and fat. This process facilitates the interaction between salt and the gut microflora. An HS environment could increase osmotic pressure in the intestine, and, in response to this change, the growth of enteric bacteria would be suppressed. This may be why the total bacteria load in the intestine showed a decreased trend after HS intake. The composition of the microbiota also changed. This may be due to different responses of the bacteria to salt and, therefore, HS intake did not uniformly suppress bacteria growth. These ‘quantitative’ and ‘qualitative’ changes acted together to cause enteric dysbiosis. Normally, in states of dysbiosis, the levels of Firmicutes were increased and the levels of Bacteroidetes were decreased. For example, obesity is associated with an increased ratio of Firmicutes/Bacteroidetes;29, 30 however, the opposite trend is also observed in many other diseases. For example, alcoholic liver disease31 and Crohn’s disease32 are linked to a decrease in the abundance of Firmicutes and an increase in Bacteroidetes, which was similar to our current finding. Therefore, the balance between Firmicutes and Bacteroidetes in the gut seems to be dependent on the physiology of the disorder. Dysbiosis is always linked to an impaired gut pathophysiology response. For example, chronic alcohol feeding induced enteric eubiosis disruption, which could cause intestinal inflammation, including TNF-α upregulation and further permeability elevation.31 In this study, we found that the immunological response characterized by immune-related gene expression was widely disrupted in both the ileum and the colon after chronic HS intake. An interesting finding was that the trend of the cytokine–cytokine receptor interaction in the ileum and the colon seemed to be the opposite. A possible explanation was the presence of enteric eubiosis. Cytokine expression and release in the gut largely depends on the enteric bacteria.33 Although bacterial composition was altered in the cecum and the mucus layer of the ileum and the colon, the consumption of an HS diet did not uniformly disrupt their composition, which means that the dysbiosis that occurred throughout the intestine was different. This difference may lead to different cytokine–cytokine receptor responses in the ileum and the colon, which further demonstrated that the immunological response in the gut was impaired after HS intake.
Dysbiosis and gut inflammation may affect each other. In the current study, dysbiosis directly induced by HS treatment would be the upstream factor for intestinal inflammation. (1) In antibiotic and HS co-treated mice, the intestinal Ifn-γ mRNA level was similar to that in control mice and markedly decreased compared with that in HS mice (Figure 5a), demonstrating that HS alone could not, but that gut microbiota could, induce intestinal inflammation; (2) gut inflammation could indeed disrupt the intestinal microbiota; however, this disruption mainly occurred during obvious inflammation or a long-term inflammatory response,34 as reported by others. In our present study, we believe that chronic HS-induced inflammation was mild and could not directly cause obvious microbiota composition disruption. Therefore, we propose that imbalanced eubiosis would be the upstream factor for intestinal inflammation. The immunological response impairment is believed to be connected to the alterations in gut barrier function. In a recent study regarding the ‘gut-brain axis’, Hsiao et al.35 reported that impaired cytokine expression during autism development is an upstream inducer of gut barrier function disruption. Similarly, intestinal permeability was markedly increased, which was accompanied by impaired cytokine expression after HS intake. As most bacteria from the intestine are unculturable, there are only a few reports describing the direct association between specific strains and gut barrier function. As far as we know, some pathogenic strains, such as enteropathogenic Escherichia coli, could directly disrupt the gut barrier.36 Other probiotics, such as Lactobacillus, are able to protect the gut barrier.37 However, the detailed mechanism is still unclear. An immunological response is believed to be the main mediator, which means that the microbiota should act on the intestinal immune system first and impaired inflammation could further disrupt tight junction expression and finally cause barrier dysfunction.38 In addition, because the gut microbiome is a complex system, different strains may act together to affect the intestine. It is also believed that gut permeability is influenced by the complex interaction among the various strains (or bacterial products) in the gut.39 In conclusion, enteric bacteria could indirectly affect the gut barrier through immunological regulation, but the direct interaction between specific strains and the gut barrier still needs to be illustrated.
Tight junctions are the main molecules that form the gut barrier. The regulation of tight junctions is based on two different levels. (1) mRNA level: the elevation of some cytokines is associated with decreased tight junction mRNA levels during disease progression,35 which could further disrupt the gut barrier. (2) Protein level: inflammatory factors could also directly disrupt tight junction proteins and promote barrier dysfunction.40 In the current study, the mRNA levels of major tight junctions were markedly decreased in the colon, which indicated that the regulation may occur at the mRNA level; decreased gene expression was able to lower the protein level, as shown in Figure 4d. In the ileum, we did not find an obvious change in the levels of mRNA for tight junctions (data not shown), but the protein levels were disrupted (Figure 4c), which suggested that the regulation occurred at the protein level. Our data clearly showed that the tight junctions were disrupted in both the ileum and the colon, but these disruptions were dependent on different mechanisms. Another potential pathway involved in gut barrier loss is the direct action of salt on intestinal epithelial cells. We cannot rule out this possibility, but as shown in the antibiotic experiment, gut leakiness, as monitored by fecal albumin content and the FD-4 penetration experiment, was almost restored in antibiotic and HS co-treated mice compared with HS-treated animals. This result indicated that the enteric microbiota had a key role in gut barrier dysfunction. The direct effects of salt on intestinal permeability still need to be studied in the future.
Disrupted gut barrier function causes bacteria translocation. Interestingly, in response to HS feeding, the bacteria appeared to ‘specifically’ translocate to the kidney, as we found no bacterial load elevation in the liver or the spleen. One possible explanation is that whole-body blood has a longer retention time in the kidney than in other tissues and, therefore, bacteria are easier to monitor. In additionally, the gut leakiness that is induced by HS was not that dramatic and the bacteria that translocated into the liver or the spleen could be eliminated immediately by local immunoreaction. All phenotypes, including gut immunological responses, leakiness, bacterial translocation and renal dysfunction, were restored by non-absorbable antibiotic treatment. Furthermore, the fecal microbiota transplantation results demonstrated that the gut microbiota could independently cause gut leakiness and renal injury, which strongly indicated that HS intake-induced enteric dysbiosis was the original trigger.
Blood pressure and renal injury are closely linked, but the association between kidney damage and hypertension is complex. It is believed that chronic renal injury could promote the development of hypertension;3 on the other hand, established hypertension is able to cause kidney injury and dysfunction.41 Therefore, the mediator for kidney injury observed in the current study may be from HS alone or from the increased blood pressure induced by HS. Although we found that SBP was increased after HS treatment, we cannot conclude that these mice developed hypertension because the absolute value was not high enough and the mean blood pressure was not altered. We therefore speculate that the renal injury and dysfunction that occurred in HS-treated mice may result from HS intake. Moreover, the elevated SBP may be at least in part due to renal dysfunction. Collectively, together with the antibiotic data, we propose that HS-induced enteric dysbiosis and gut barrier dysfunction is the upstream trigger for kidney injury, and renal dysfunction could further promote blood pressure elevation.
In summary, our study provides a novel direction in the investigation of the pathogenesis of salt-related hypertension by employing the microbiota-dependent ‘gut-kidney axis’ theory. Future work will focus on the connection between enteric bacteria and the onset of salt-induced hypertension, will specify the strain of bacteria or the metabolic pathway in the bacteria that contributes to the gut and renal pathological changes induced by salt, and will provide a potential therapeutic target for hypertension intervention based on our novel finding in the ‘Gut-Kidney Axis’.
References
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J . Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365: 217–223.
Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW et al. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 2013; 61: 457–464.
Ruilope LM, Bakris GL . Renal function and target organ damage in hypertension. Eur Heart J 2011; 32: 1599–1604.
Dahl LK, Leitl G, Heine M . Influence of dietary potassium and sodium/potassium molar ratios on the development of salt hypertension. J Exp Med 1972; 136: 318–330.
Kobori H, Nishiyama A, Abe Y, Navar LG . Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 2003; 41: 592–597.
Yu HC, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME et al. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation 1998; 98: 2621–2628.
Antonios TF, MacGregor GA . Salt—more adverse effects. Lancet 1996; 348: 250–251.
Chen P, Schnabl B . Host-microbiome interactions in alcoholic liver disease. Gut Liver 2014; 8: 237–241.
Schnabl B, Brenner DA . Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014; 146: 1513–1524.
Schnabl B . Linking intestinal homeostasis and liver disease. Curr Opin Gastroenterol 2013; 29: 264–270.
Chen P, Torralba M, Tan J, Embree M, Zengler K, Stärkel P et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology 2015; 148: 203–214.
Wang L, Fouts DE, Stärkel P, Hartmann P, Chen P, Llorente C et al. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe 2016; 19: 227–239.
Yin J, Prabhakar M, Wang S, Liao SX, Peng X, He Y et al. Different dynamic patterns of β-lactams, quinolones, glycopeptides and macrolides on mouse gut microbial diversity. PLoS ONE 2015; 10: e0126712.
Chen P, Han Z, Yang P, Zhu L, Hua Z, Zhang J . Loss of clock gene mPer2 promotes liver fibrosis induced by carbon tetrachloride. Hepatol Res 2010; 40: 1117–1127.
Li Q, Cao C, Zhang C, Zheng S, Wang Z, Wang L et al. The identification of Cucumis sativus Glabrous 1 (CsGL1) required for the formation of trichomes uncovers a novel function for the homeodomain-leucine zipper I gene. J Exp Bot 2015; 66: 2515–2526.
Yu Y, Zeng L, Yan Z, Liu T, Sun K, Zhu T et al. Identification of Ramie genes in response to Pratylenchus coffeae infection challenge by digital gene expression analysis. Int J Mol Sci 2015; 16: 21989–22007.
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B . Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 2008; 5: 621–628.
Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem 2015; 290: 5647–5660.
Andersson AF, Lindberg M, Jakobsson H, Bäckhed F, Nyrén P, Engstrand L . Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS ONE 2008; 3: e2836.
Cash HL, Whitham CV, Behrendt CL, Hooper LV . Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006; 313: 1126–1130.
Yu LC, Shih YA, Wu LL, Lin YD, Kuo WT, Peng WH et al. Enteric dysbiosis promotes antibiotic-resistant bacterial infection: systemic dissemination of resistant and commensal bacteria through epithelial transcytosis. Am J Physiol Gastrointest Liver Physiol 2014; 307: G824–G835.
Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011; 53: 96–105.
Su L, Nalle SC, Shen L, Turner ES, Singh G, Breskin LA et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology 2013; 145: 407–415.
LaTuga MS, Ellis JC, Cotton CM, Goldberg RN, Wynn JL, Jackson RB et al. Beyond bacteria: a study of the enteric microbial consortium in extremely low birth weight infants. PLoS ONE 2011; 6: e27858.
Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG . Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 1995; 108: 218–224.
Ghosh SS, Bie J, Wang J, Ghosh S . Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR−/− mice—role of intestinal permeability and macrophage activation. PLoS ONE 2014; 9: e108577.
Muskiet MH, Smits MM, Morsink LM, Diamant M . The gut-renal axis: do incretin-based agents confer renoprotection in diabetes? Nat Rev Nephrol 2014; 10: 88–103.
Wu IW, Hsu KH, Lee CC, Sun CY, Hsu HJ, Tsai CJ et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant 2011; 26: 938–947.
Verdam FJ, Fuentes S, de Jonge C, Zoetendal EG, Erbil R, Greve JW et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity (Silver Spring) 2013; 21: E607–E615.
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE et al. A core gut microbiome in obese and lean twins. Nature 2009; 457: 480–484.
Chen P, Stärkel P, Turner JR, Ho SB, Schnabl B . Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology 2015; 61: 883–894.
Man SM, Kaakoush NO, Mitchell HM . The role of bacteria and pattern-recognition receptors in Crohn's disease. Nat Rev Gastroenterol Hepatol 2011; 8: 152–168.
Haller D, Bode C, Hammes WP, Pfeifer AM, Schiffrin EJ, Blum S . Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 2000; 47: 79–87.
Duboc H, Rajca S, Rainteau D, Benarous D, Maubert MA, Quervain E et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 2013; 62: 531–539.
Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013; 155: 1451–1463.
Yuhan R, Koutsouris A, Savkovic SD, Hecht G . Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology 1997; 113: 1873–1882.
Mangell P, Nejdfors P, Wang M, Ahrné S, Weström B, Thorlacius H et al. Lactobacillus plantarum 299v inhibits Escherichia coli-induced intestinal permeability. Dig Dis Sci 2002; 47: 511–516.
Wu LL, Peng WH, Kuo WT, Huang CY, Ni YH, Lu KS et al. Commensal bacterial endocytosis in epithelial cells is dependent on myosin light chain kinase-activated brush border fanning by interferon-γ. Am J Pathol 2014; 184: 2260–2274.
Eutamene H, Lamine F, Chabo C, Theodorou V, Rochat F, Bergonzelli GE et al. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr 2007; 137: 1901–1907.
Ma TY, Boivin MA, Ye D, Pedram A, Said HM . Mechanism of TNF-alpha modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol 2005; 288: G422–G430.
Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J et al. Renal injury from angiotensin II-mediated hypertension. Hypertension 1992; 19: 464–474.
Acknowledgements
We are grateful to Prof Jerrold Turner (Departments of Pathology and Medicine, Brigham and Women’s Hospital, Harvard Medical School, USA) for expert guidance in the gut barrier study. We thank Prof Zhixing Pan (Medical University of Ohio, USA) for the discussion. This study was supported in part by the Startup Foundation for Advanced Talents of Southern Medical University, the National Science Foundation of China (grant 31500952) and the Natural Science Funds for Distinguished Young Scholar of Guangdong province (2016A030306043) to PC. Key Scientific and Technological Program of Guangzhou City (No 201607020016) to YJ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Hu, J., Luo, H., Wang, J. et al. Enteric dysbiosis-linked gut barrier disruption triggers early renal injury induced by chronic high salt feeding in mice. Exp Mol Med 49, e370 (2017). https://doi.org/10.1038/emm.2017.122
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2017.122
This article is cited by
-
Hydrangea paniculata coumarins attenuate experimental membranous nephritis by bidirectional interactions with the gut microbiota
Communications Biology (2023)
-
Fecal microbiota transplantation and replenishment of short-chain fatty acids protect against chronic cerebral hypoperfusion-induced colonic dysfunction by regulating gut microbiota, differentiation of Th17 cells, and mitochondrial energy metabolism
Journal of Neuroinflammation (2022)
-
Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via Toll-like receptor 2 signaling
Microbiome (2022)
-
Higher bacterial DNAemia can affect the impact of a polyphenol-rich dietary pattern on biomarkers of intestinal permeability and cardiovascular risk in older subjects
European Journal of Nutrition (2022)
-
Gut microbiota accelerates cisplatin-induced acute liver injury associated with robust inflammation and oxidative stress in mice
Journal of Translational Medicine (2021)