Abstract
Nephronophthisis-related ciliopathy (NPHP-RC) is a common genetic cause of end-stage renal failure during childhood and adolescence and exhibits an autosomal recessive pattern of inheritance. Genetic diagnosis is quite limited owing to genetic heterogeneity in NPHP-RC. We designed a novel approach involving the step-wise screening of Sanger sequencing and targeted exome sequencing for the genetic diagnosis of 55 patients with NPHP-RC. First, five NPHP-RC genes were analyzed by Sanger sequencing in phenotypically classified patients. Known pathogenic mutations were identified in 12 patients (21.8%); homozygous deletions of NPHP1 in 4 juvenile nephronophthisis patients, IQCB1/NPHP5 mutations in 3 Senior–Løken syndrome patients, a CEP290/NPHP6 mutation in 1 Joubert syndrome patient, and TMEM67/MKS3 mutations in 4 Joubert syndrome patients with liver involvement. In the remaining undiagnosed patients, we applied targeted exome sequencing of 34 ciliopathy-related genes to detect known pathogenic mutations in 7 (16.3%) of 43 patients. Another 18 likely damaging heterozygous variants were identified in 13 NPHP-RC genes in 18 patients. In this study, we report a variety of pathogenic and candidate mutations identified in 55 patients with NPHP-RC in Korea using a step-wise application of two genetic tests. These results support the clinical utility of targeted exome sequencing to resolve the issue of allelic and genetic heterogeneity in NPHP-RC.
Similar content being viewed by others
Introduction
Nephronophthisis (NPHP) should be included in the differential diagnosis of children or adolescents presenting with chronic renal failure (CRF) of unknown etiology, because NPHP is the most common (accounting for ~3%) monogenic autosomal recessive cause of CRF in this age group.1, 2, 3 NPHP is clinically characterized by anemia and growth retardation due to impaired renal function as well as polyuria/nocturia and polydipsia due to decreased renal concentrating ability. Urinalysis often appears normal,4 and blood pressure is typically not high. Commonly, ultrasonography reveals normal-sized or relatively small kidneys that are distinctive from autosomal recessive or autosomal dominant polycystic kidney disease (ARPKD or ADPKD)5 and lack corticomedullary differentiation. NPHP is often accompanied by defects in other organs and tissues, for example, the retina, cerebellum and liver, such as in Joubert syndrome, Senior–Løken syndrome and Meckel–Gruber syndrome (MKS). A collective term, NPHP-related ciliopathy (NPHP-RC), is used to describe this group of diseases, because most of the causative genes in these disorders encode proteins that have a role in the cilium.6, 7, 8
A genetic diagnosis is required for a definitive diagnosis of NPHP-RC, because the clinical features of patients with NPHP-RC are rather non-specific, and their symptoms overlap significantly.8 The most common genetic cause of NPHP is a large deletion of NPHP1,9, 10, 11 which is noted in >20% of patients with NPHP, whereas other genes contribute less than 2–3%.7 Sanger sequencing of commonly mutated NPHP genes detects mutations in less than one-third of patients.8 In addition, the number of NPHP-RC-related genes has been rapidly increasing, with 20 genes having NPHP as part of their gene name at the time of writing, especially after the introduction of next-generation sequencing (NGS) techniques.12, 13, 14 Therefore, the genetic diagnosis of NPHP-RC based on an ‘educated guess at the best candidate gene’ is now changing to adopting high-throughput genome analysis techniques. Through NGS screening of known and candidate genes, a genetic diagnosis can be obtained efficiently in more patients, and novel causative genes can be identified. However, this technology is still evolving, and it is relatively costly and requires advanced bioinformatics support for handling large quantities of data. Therefore, the combination of both traditional Sanger sequencing and NGS might be more practical in obtaining a genetic diagnosis of NPHP-RC or other genetically heterogeneous groups of disorders.
We report here the use of a stratified two-step procedure of Sanger sequencing of selected genes followed by targeted exome sequencing of 34 disease-related genes, which led to a genetic diagnosis for 19 (34.5%) of our cohort of 55 Korean patients with NPHP-RC clinical diagnoses.
Materials and methods
Study population
This study was approved by the independent review board of Seoul National University Hospital (H-0812-002-264), and only those patients who provided written informed consent were screened and included in this study. Patients who were clinically diagnosed with NPHP were included.15 Presentation with incidentally identified CRF in the first three decades of life without evidence of previous renal damage was typical. Other causes of CRF were excluded by past medical history and imaging of the urinary tract in the majority of the cases. Patients with a clinical diagnosis of ADPKD were excluded. One patient (J-86) was included owing to typical findings of Joubert syndrome (cerebellar vermis aplasia, Leber congenital amaurosis and mental retardation) without renal involvement. We performed fundus examinations and abdominal sonography to evaluate the intra-abdominal organs. Renal ultrasonographic findings of increased echogenicity (normal or slightly decreased size for age) and corticomedullary differentiation loss were considered to be typical for NPHP-RC.5 For those individuals with developmental delay and neurological problems, we requested brain imaging. For some patients (n=15), renal pathology was also obtained, which exhibited typical findings of chronic tubular interstitial disease. This study adheres to the Declaration of Helsinki.
The patients were classified according to their age at the time of end-stage renal disease as infantile when younger than 5 years old (n=11) and juvenile when older (n=44). Combinations of NPHP with cerebellar vermis hypoplasia or aplasia were designated as Joubert syndrome (n=8). Retinal involvement with retinitis pigmentosa (RP) or Leber’s congenital amaurosis was designated as Senior–Løken syndrome (n=13), and those patients with multiple problems without cerebellar hypo/aplasia were designated as MKS-like (n=3).
Sanger sequencing
The most probable candidate genes were selected for Sanger sequencing based on known mutation frequencies and specific clinical phenotypes (Figure 1). Peripheral blood mononuclear cells were collected from the patients, and genomic DNA was obtained using a QIA amp DNA Blood Mini Kit (Qiagen, Hilden, Germany). All of the patients (n=55) were screened for large deletions of NPHP1, which is the most common mutation in NPHP, by amplifying each of the exons using PCR; a failure of amplification was considered to be a total homozygous deletion of the NPHP1 gene.10, 16 Among those patients without large deletions of NPHP1, infantile NPHP patients were tested for INVS/NPHP2 mutations (n=11).17 The selection of genes according to phenotype was as follows: IQCB1/NPHP5 for RP (n=19);18 CEP290/NPHP6 for Joubert syndrome (n=8);19 and TMEM67/MKS3 for hepatic fibrosis (n=7).20, 21
Strategy for Sanger sequencing. All of the patients were screened for complete deletions of NPHP1, which is the most common mutation in NPHP. Among those without complete NPHP1 deletion, infant NPHP patients were tested for INVS/NPHP2 mutations. IQCB1/NPHP5, CEP290/NPHP6, and TMEM67/MKS3/NPHP11 were tested according to the phenotypes of the patients. Crbll, cerebellar; F, female; M, male; NPHP, nephronophthisis; RP, retinitis pigmentosa; SNHL, sensory neural hearing loss.
Targeted exome sequencing
We designed customized targeted exome capture using a SeqCap EZ kit (Roche NimbleGen, Madison, WI, USA) for 34 genes related to NPHP-RC, Bardet–Biedl syndrome and ARPKD (Supplementary Table S1). After capturing the target sequences, the DNA libraries were amplified and sequenced using Illumina HiSeq2000 (Illumina, San Diego, CA, USA).
Alignment, coverage calculation and variant detection
Reads were aligned to the UCSC hg19 reference genome using BWA-0.6.1 with default settings22 for single-nucleotide variation (SNV)/insertion and deletion (indel) detection, and duplicate reads were removed. Variants were identified using Unified Genotyper in GATK-1.3.23 A Perl script and Annovar were used to annotate variants and search the known SNPs and indels from dbSNP v135 and the 1000 Genomes project data (drafted February 2012). Coverage and depth were calculated using the GATK DepthOfCoverage analysis. The significance of variants was assessed in silico using MutationTaster, PolyPhen-2, SIFT and FATHMM. Those variants predicted to be disease causing or not tolerated by two or more programs were considered to be pathogenic mutations.24, 25, 26, 27 Conservation scores (Phylo-P) for each mutated nucleotide were also considered.28 Prediction of alternative splicing by intronic variation was performed using NetGene2 (http://www.cbs.dtu.dk/services/NetGene2/), Ex-skip (http://ex-skip.img.cas.cz/), and BDGP acceptor site prediction (http://www.fruitfly.org/seq_tools/other.html).
Variant calls were obtained using the following filter parameter: minimum base quality of 17 (the default value for the GATK Unified Genotyper). A minimum variant count of 10 was applied for potential truncating mutations (nonsense, frameshift and obligatory splice-site mutations), and non-synonymous missense variants were filtered by a minimum count of 3. Synonymous variants and common dbSNP (v135) variants with a population allele frequency >1% were excluded.
All of the candidate mutations were validated by Sanger sequencing. When available, we verified their co-segregation in the family (J-61 and J-50).
Results
Clinical features of NPHP-RC patients
We recruited 55 unrelated Korean patients with a clinical diagnosis of NPHP (M:F=34:21) who were referred to our laboratory for genetic diagnosis (Supplementary Table S1). The mean age of the patients at end-stage renal disease was 9.0±5.1 years old (median, 8.3 years; range, 0.6–17.9 years). Renal histology from 15 patients exhibited typical findings of tubulointerstitial nephropathy. Nineteen patients (34%) had eye involvement of RP or Leber’s congenital amaurosis, eight patients (14.5%) had a molar tooth sign in the brainstem on brain imaging and seven patients (12.7%) exhibited hepatic fibrosis. Eight patients (14.5%) had siblings with similar symptoms; however, none of their parents had CRF.
First step: Sanger sequencing for genetic diagnosis
As a first step in the stratified approach to determine a genetic diagnosis of NPHP, we selected and screened the patients by Sanger sequencing for candidate genes (Figure 1). A homozygous deletion of NPHP1 was detected in four patients (7.3% (confidence interval (CI, 95%) 0.4–14.2%), and 17% of patients exhibited juvenile isolated NPHP without other organ involvement (n=23); Table 1, in bold). Three (15.8%) of 19 NPHP patients with RP or Leber’s congenital amaurosis (Senior- Løken syndrome, marked with ♣ in Figure 1) carried a common pathogenic homozygous mutation (c.1522_1523dupGA, p.Ala509Lysfs*3) in the IQCB1/NPHP5 genes.29 In screening NPHP patients with cerebellar vermis hypotrophy/atrophy involvement (n=8, marked with ♦ in Figure 1), one familial case of Joubert syndrome had a compound heterozygous mutation in CEP290/NPHP6, a known pathogenic c.1666delA, p.Ile556Phefs*17,30 and an SNV (c.6011-12T>A) that is predicted to cause alternative splicing.31 Four of seven NPHP patients with hepatic fibrosis (marked with ♣ in Figure 1) carried compound heterozygous mutations in TMEM67/MKS3/JBSTS6, a frameshift, truncating mutation and an SNV.32, 33, 34 All four of these patients had Joubert syndrome and congenital hepatic fibrosis compatible with COACH (cerebellar vermis hypoplasia/aplasia, oligophrenia, congenital ataxia, ocular coloboma and hepatic fibrosis) syndrome.35 In total, Sanger sequencing detected genetic aberrations in 12 patients (21.8%) with a clinical diagnosis of NPHP-RC.
Second step: Targeted exome sequencing in patients with NPHP-RC
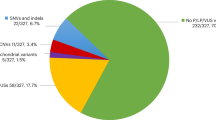
Targeted exome sequencing of 34 NPHP-RC-related genes was applied in 43 patients in whom a genetic diagnosis was not obtained by our first step of Sanger sequencing. We obtained an average 21.8 Mb of mapped data per individual for ~166 kb of targeting and flanking regions (Supplementary Table S2). The mean read depth was 131±55 and 95.4% of the captured target exons exhibited >10-fold coverage. Most likely azpathogenic mutations of various types were identified in 7 of 43 cases (16%) in six genes, including NPHP1, NPHP3, NPHP4, SDCCAG8/NPHP10, TTC21B/NPHP12 and PKHD1 (Figure 2). In three cases (K-8, J-84, K-9, Table 2), a heterozygous known pathogenic mutation in BBS4 was identified that could not explain the genetic pathogenesis but might explain a mild phenotype resembling BBS, as previously reported.36, 37 Heterozygous potentially damaging mutations/variations in at least one of the target NPHP-RC genes were identified in additional 15 patients, including one truncating mutation of NPHP3 in two patients with isolated juvenile NPHP. In the remaining 18 patients, no significant variations of the target genes were identified. In total, we identified most likely pathogenic mutations in nine NPHP-RC-related genes in 19 (34.5%) of 55 Asian NPHP-RC patients using a two-step genetic diagnosis (Figure 2), where the frequency of homozygous deletion of NPHP1 is relatively low (7.3%, CI 0.4–14.2).
Results of genetic diagnosis for NPHP-RC. One-third (n=19; 34.5%) of the patients with clinical diagnoses of NPHP-RC obtained a genetic diagnosis by two-step genetic diagnosis using Sanger sequencing (n=12, 21.8%) and targeted exome sequencing (n=7; 12.7%). Four patients with homozygous total deletion of NPHP1, three with IQCB1/NPHP5, one with CEP290/NPHP6, and four with TMEM67/MKS3/NPHP11 were detected using Sanger sequencing. Mutations of other genes were detected using targeted exome sequencing. In addition, heterozygous mutations in NPHP-RC genes were detected in 13 patients (23.6%). NPHP-RC, nephronophthisis-related ciliopathy.
Discussion
The genetic causes of several Mendelian diseases, such as NPHP-RC, RP, and non-syndromic hearing loss, are heterogeneous. For these diseases, providing a precise diagnosis is often difficult until pathogenic mutations are identified. In the present study, we obtained a genetic diagnosis in one-third of patients with a clinical diagnosis of NPHP-RC with a two-step genetic diagnosis using Sanger sequencing followed by high-throughput mutation analysis using NGS after a custom DNA-capture procedure. For two patients (K-7 and O-463), mutation analysis led to correction of their diagnoses from NPHP to ARPKD. Patients with ARPKD may have normal-sized kidneys and therefore may be misdiagnosed with NPHP, as shown here. In addition, additional heterozygous mutations were identified in candidate genes from NGS (data not shown), implying that further study of the respective genes or other closely related genes would enhance the efficacy of genetic diagnosis of NPHP-RC. For the definitive genetic diagnosis of NPHP-RC patients, extensive analysis of trio or family studies of NPHP-RC will be followed to understand the penetrance of genetic alterations and recurrence in additional patients. Systemic functional studies on the variant proteins will also be required to understand the effects of genetic alterations.
Traditionally, genetic diagnosis has been obtained by Sanger sequencing of the best candidate genes based on disease phenotype and frequency. The same approach was applied for our NPHP-RC patients as our first step, and 22% of the patients were given genetic diagnosis with a fair genotype–phenotype correlation. All three patients with congenital blindness due to Leber’s congenital amaurosis were shown to have an identical homozygous indel mutation in IQCB1/NPHP5 (5.5% (CI 0–11.5%) of the total population), suggesting a founder effect. Four patients with Joubert syndrome, RP, hepatic fibrosis and developmental delay were found to have TMEM67/MKS3/JBSTS6 mutations (7.3% (CI 0.4–11.5%) of the total population). These four patients had a similar appearance to each other, with rectangular faces, square jaws, and amiable natures despite mental retardation. Another interesting finding at this step was that the proportion of NPHP1 mutations in these patients was relatively lower than reported.7, 8, 12 It is not clear whether this difference reflects ethnic characteristics of the study populations or is simply derived from a selection bias of this study given the small number of participants. Nonetheless, a majority of the patients were not given a definitive diagnosis, similar to that reported in the literature.7, 8, 12, 38 Those with an atypical phenotype would have been misclassified in this step, and their causative genes would not have been assessed. Therefore, we introduced NGS technology as our second step.
For better cost-effectiveness, we chose targeted exome sequencing (TES) instead of whole-genome sequencing or whole-exome sequencing. TES for disease-related genes with high read depth enables multiplex screening of candidate genes.38 In addition to a well-defined set of known NPHP-RC genes known at the time of study design, we included an extended set of cilia-related genes in our TES (Supplementary Table S2), including ARPKD and genes of interest to the authors. Among the variants detected in our second step of genetic diagnosis, only those that might explain the phenotype were selected as the most likely pathogenic mutations (n=13, Table 2); four known pathogenic mutations, five frameshift or truncating mutations and four missense mutations were predicted to be damaging. The possibility of large deletions or duplications was also considered, and total deletion of one NPHP1 allele was detected in one patient (J-39) in whom the other NPHP1 allele exhibited a heterozygous frame shift mutation. Other than this case (J-39), no genetic diagnosis was made involving the five genes selected for our first step. Given that those with known genetic defects were excluded at this second step, the efficiency of TES could not be assessed. Overall, this second step of genetic diagnosis mapped genetic aberrations in known NPHP-RC genes or PKHD1 in 16% (7 of 43) of the patients. Our results reflect a similar mutation-detection rate to that reported by Halbritter et al.38, where a molecular diagnosis was obtained in 12% of patients using high-throughput mutation screening of 13 NPHP genes in a large population of NPHP-RC patients.
This study has several shortcomings. The number of the patients is small compared with previous reports,38, 39 and segregation analysis was not available in the majority of the cases due to the inability to obtain a sample from the parents. In addition, functional studies of ‘most likely pathogenic’ novel mutations have not been performed to date. In addition, given that the coverage of TES of this study was not perfect (Supplementary Table S3), a second mutation might not be sequenced, thus warranting Sanger sequencing of the respective genes with single candidate variants, Unfortunately, further analysis was not possible in this study because those with single variants were lost to follow-up. Above all, the set of genes used for TES reflects our knowledge at the time of study design; therefore, newly discovered genes, such as ZNF423,40 WDR19,41 ANKS6,42 IFT172,43 CEP83 (ref. 44) and DCDC2,45 were not included. On the basis of this study, we are currently developing our next set of TES by incorporating the recent discovery of NPHP-RC. Improvements in NSG technology are expected to increase the rate of genetic diagnosis.
Although the number of patients of this study was not sufficiently large to determine the distribution of genetic aberration types and loci, this finding helps to delineate the characteristics of Korean or Asian NPHP, which enables a more efficient genetic diagnosis of NPHP-RC in this population. On the basis of this study, we are currently assessing the genetic aberrations of our NPHP-RC patients as follows. Gel electrophoresis of PCR products of NPHP1 is performed as the first step (step 1), and then one or two particular mutations or genes are analyzed if the patient has distinctive extra-renal findings, such as congenital blindness with c.1523_1524insGA of IQCB1/NPHP5 or COACH syndrome with a TMEM67/MKS3/JBSTS6 mutation (step 2). If genetic diagnosis is not obtained with these first two steps, then the TES steps in (step 3) might be replaced by whole-exome sequencing or whole-genome sequencing in the near future. With expanding collective knowledge and rapidly evolving technology, both steps 2 and 3 are expected to simultaneously become more diverse and more precise, thus yielding a better genetic diagnosis of NPHP-RC.
References
Steele BT, Lirenman DS, Beattie CW . Nephronophthisis. Am J Med 1980; 68: 531–538.
Avner ED, Harmon WE, Niaudet P, Yoshikawa N, Emma F, Goldstein SL . Pediatric nephrology. Springer: Heidelberg, Germany, 2016.
Hamiwka LA, Midgley JP, Wade AW, Martz KL, Grisaru S . Outcomes of kidney transplantation in children with nephronophthisis: an analysis of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Registry. Pediatr Transplant 2008; 12: 878–882.
Hirano D, Fujinaga S, Ohtomo Y, Nishizaki N, Hara S, Murakami H et al. Nephronophthisis cannot be detected by urinary screening program. Clin Pediatr (Phila) 2012; 52: 759–761.
Blowey DL, Querfeld U, Geary D, Warady BA, Alon U . Ultrasound findings in juvenile nephronophthisis. Pediatr Nephrol 1996; 10: 22–24.
Hildebrandt F, Otto E . Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet 2005; 6: 928–940.
Chaki M, Hoefele J, Allen SJ, Ramaswami G, Janssen S, Bergmann C et al. Genotype-phenotype correlation in 440 patients with NPHP-related ciliopathies. Kidney Int 2011; 80: 1239–1245.
Hildebrandt F, Attanasio M, Otto E . Nephronophthisis: disease mechanisms of a ciliopathy. J Am Soc Nephrol 2009; 20: 23–35.
Nothwang HG, Stubanus M, Adolphs J, Hanusch H, Vossmerbaumer U, Denich D et al. Construction of a gene map of the nephronophthisis type 1 (NPHP1) region on human chromosome 2q12-q13. Genomics 1998; 47: 276–285.
Hildebrandt F, Otto E, Rensing C, Nothwang HG, Vollmer M, Adolphs J et al. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet 1997; 17: 149–153.
Soliman NA, Hildebrandt F, Otto EA, Nabhan MM, Allen SJ, Badr AM et al. Clinical characterization and NPHP1 mutations in nephronophthisis and associated ciliopathies: a single center experience. Saudi J Kidney Dis Transpl 2012; 23: 1090–1098.
Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell 2011; 145: 513–528.
Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C et al. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet 2010; 42: 840–850.
Wolf MT . Nephronophthisis and related syndromes. Curr Opin Pediatr 2015; 27: 201–211.
Hildebrandt F, Strahm B, Nothwang HG, Gretz N, Schnieders B, Singh-Sawhney I et al. Molecular genetic identification of families with juvenile nephronophthisis type 1: rate of progression to renal failure. Kidney Int 1997; 51: 261–269.
Al-Romaih KI, Genovese G, Al-Mojalli H, Al-Othman S, Al-Manea H, Al-Suleiman M et al. Genetic diagnosis in consanguineous families with kidney disease by homozygosity mapping coupled with whole-exome sequencing. Am J Kidney Dis 2011; 58: 186–195.
Otto EA, Schermer B, Obara T, O'Toole JF, Hiller KS, Mueller AM et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 2003; 34: 413–420.
Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet 2005; 37: 282–288.
Sayer JA, Otto EA, O'Toole JF, Nurnberg G, Kennedy MA, Becker C et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet 2006; 38: 674–681.
Baala L, Romano S, Khaddour R, Saunier S, Smith UM, Audollent S et al. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet 2007; 80: 186–194.
Otto EA, Tory K, Attanasio M, Zhou W, Chaki M, Paruchuri Y et al. Hypomorphic mutations in meckelin (MKS3/TMEM67) cause nephronophthisis with liver fibrosis (NPHP11). J Med Genet 2009; 46: 663–670.
Min BJ, Kim N, Chung T, Kim OH, Nishimura G, Chung CY et al. Whole-exome sequencing identifies mutations of KIF22 in spondyloepimetaphyseal dysplasia with joint laxity, leptodactylic type. Am J Hum Genet 2011; 89: 760–766.
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010; 20: 1297–1303.
Shihab HA, Gough J, Cooper DN, Stenson PD, Barker GL, Edwards KJ et al. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat 2013; 34: 57–65.
Adzhubei I, Jordan DM, Sunyaev SR . Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet 2013; 7: 20.
Kumar P, Henikoff S, Ng PC . Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 2009; 4: 1073–1081.
Schwarz JM, Rodelsperger C, Schuelke M, Seelow D . MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 2010; 7: 575–576.
Gilissen C, Hoischen A, Brunner HG, Veltman JA . Disease gene identification strategies for exome sequencing. Eur J Hum Genet 2012; 20: 490–497.
Estrada-Cuzcano A, Koenekoop RK, Coppieters F, Kohl S, Lopez I, Collin RW et al. IQCB1 mutations in patients with leber congenital amaurosis. Invest Ophthalmol Vis Sci 2011; 52: 834–839.
Brancati F, Barrano G, Silhavy JL, Marsh SE, Travaglini L, Bielas SL et al. CEP290 mutations are frequently identified in the oculo-renal form of Joubert syndrome-related disorders. Am J Hum Genet 2007; 81: 104–113.
Tsurusaki Y, Kobayashi Y, Hisano M, Ito S, Doi H, Nakashima M et al. The diagnostic utility of exome sequencing in Joubert syndrome and related disorders. J Hum Genet 2013; 58: 113–115.
Szymanska K, Berry I, Logan CV, Cousins SR, Lindsay H, Jafri H et al. Founder mutations and genotype-phenotype correlations in Meckel-Gruber syndrome and associated ciliopathies. Cilia 2012; 1: 18.
Brancati F, Iannicelli M, Travaglini L, Mazzotta A, Bertini E, Boltshauser E et al. MKS3/TMEM67 mutations are a major cause of COACH Syndrome, a Joubert Syndrome related disorder with liver involvement. Hum Mutat 2009; 30: E432–E442.
Doherty D, Parisi MA, Finn LS, Gunay-Aygun M, Al-Mateen M, Bates D et al. Mutations in 3 genes (MKS3, CC2D2A and RPGRIP1L) cause COACH syndrome (Joubert syndrome with congenital hepatic fibrosis). J Med Genet 2010; 47: 8–21.
Gentile M, Di Carlo A, Susca F, Gambotto A, Caruso ML, Panella C et al. COACH syndrome: report of two brothers with congenital hepatic fibrosis, cerebellar vermis hypoplasia, oligophrenia, ataxia, and mental retardation. Am J Med Genet 1996; 64: 514–520.
Hoskins BE, Thorn A, Scambler PJ, Beales PL . Evaluation of multiplex capillary heteroduplex analysis: a rapid and sensitive mutation screening technique. Hum Mutat 2003; 22: 151–157.
Karmous-Benailly H, Martinovic J, Gubler MC, Sirot Y, Clech L, Ozilou C et al. Antenatal presentation of Bardet-Biedl syndrome may mimic Meckel syndrome. Am J Hum Genet 2005; 76: 493–504.
Halbritter J, Porath JD, Diaz KA, Braun DA, Kohl S, Chaki M et al. Identification of 99 novel mutations in a worldwide cohort of 1,056 patients with a nephronophthisis-related ciliopathy. Hum Genet 2013; 132: 865–884.
Halbritter J, Diaz K, Chaki M, Porath JD, Tarrier B, Fu C et al. High-throughput mutation analysis in patients with a nephronophthisis-associated ciliopathy applying multiplexed barcoded array-based PCR amplification and next-generation sequencing. J Med Genet 2012; 49: 756–767.
Chaki M, Airik R, Ghosh AK, Giles RH, Chen R, Slaats GG et al. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell 2012; 150: 533–548.
Bredrup C, Saunier S, Oud MM, Fiskerstrand T, Hoischen A, Brackman D et al. Ciliopathies with skeletal anomalies and renal insufficiency due to mutations in the IFT-A gene WDR19. Am J Hum Genet 2011; 89: 634–643.
Hoff S, Halbritter J, Epting D, Frank V, Nguyen TM, van Reeuwijk J et al. ANKS6 is a central component of a nephronophthisis module linking NEK8 to INVS and NPHP3. Nat Genet 2013; 45: 951–956.
Halbritter J, Bizet AA, Schmidts M, Porath JD, Braun DA, Gee HY et al. Defects in the IFT-B component IFT172 cause Jeune and Mainzer-Saldino syndromes in humans. Am J Hum Genet 2013; 93: 915–925.
Failler M, Gee HY, Krug P, Joo K, Halbritter J, Belkacem L et al. Mutations of CEP83 cause infantile nephronophthisis and intellectual disability. Am J Hum Genet 2014; 94: 905–914.
Schueler M, Braun DA, Chandrasekar G, Gee HY, Klasson TD, Halbritter J et al. DCDC2 mutations cause a renal-hepatic ciliopathy by disrupting Wnt signaling. Am J Hum Genet 2015; 96: 81–92.
Otto E, Hoefele J, Ruf R, Mueller AM, Hiller KS, Wolf MT et al. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet 2002; 71: 1161–1167.
Denamur E, Delezoide AL, Alberti C, Bourillon A, Gubler MC, Bouvier R et al. Genotype-phenotype correlations in fetuses and neonates with autosomal recessive polycystic kidney disease. Kidney Int 2010; 77: 350–358.
Hao X, Liu S, Dong Q, Zhang H, Zhao J, Su L . Whole exome sequencing identifies recessive PKHD1 mutations in a Chinese twin family with Caroli disease. PLoS One 2014; 9: e92661.
Sharp AM, Messiaen LM, Page G, Antignac C, Gubler MC, Onuchic LF et al. Comprehensive genomic analysis of PKHD1 mutations in ARPKD cohorts. J Med Genet 2005; 42: 336–349.
Kroes HY, van Zon PH, Fransen van de Putte D, Nelen MR, Nievelstein RJ, Wittebol-Post D et al. DNA analysis of AHI1, NPHP1 and CYCLIN D1 in Joubert syndrome patients from the Netherlands. Eur J Med Genet 2008; 51: 24–34.
Hichri H, Stoetzel C, Laurier V, Caron S, Sigaudy S, Sarda P et al. Testing for triallelism: analysis of six BBS genes in a Bardet-Biedl syndrome family cohort. Eur J Hum Genet 2005; 13: 607–616.
Acknowledgements
This study was supported by a grant (HI12C0014) from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Kang, H., Lee, H., Ahn, Y. et al. Targeted exome sequencing resolves allelic and the genetic heterogeneity in the genetic diagnosis of nephronophthisis-related ciliopathy. Exp Mol Med 48, e251 (2016). https://doi.org/10.1038/emm.2016.63
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2016.63
This article is cited by
-
Multigenerational effect of maternal bisphenol A exposure on DNA methylation in F1 sperm
Molecular & Cellular Toxicology (2023)
-
Comprehensive genetic analysis using next-generation sequencing for the diagnosis of nephronophthisis-related ciliopathies in the Japanese population
Journal of Human Genetics (2022)
-
Novel findings from family-based exome sequencing for children with biliary atresia
Scientific Reports (2021)
-
Novel compound heterozygous TMEM67 variants in a Vietnamese family with Joubert syndrome: a case report
BMC Medical Genetics (2020)
-
A novel homozygous ARL13B variant in patients with Joubert syndrome impairs its guanine nucleotide-exchange factor activity
European Journal of Human Genetics (2017)