Abstract
Cell therapy using stem cells has produced therapeutic benefits in animal models of COPD. Secretory mediators are proposed as one mechanism for stem cell effects because very few stem cells engraft after injection into recipient animals. Recently, nanovesicles that overcome the disadvantages of natural exosomes have been generated artificially from cells. We generated artificial nanovesicles from adipose-derived stem cells (ASCs) using sequential penetration through polycarbonate membranes. ASC-derived artificial nanovesicles displayed a 100 nm-sized spherical shape similar to ASC-derived natural exosomes and expressed both exosomal and stem cell markers. The proliferation rate of lung epithelial cells was increased in cells treated with ASC-derived artificial nanovesicles compared with cells treated with ASC-derived natural exosomes. The lower dose of ASC-derived artificial nanovesicles had similar regenerative capacity compared with a higher dose of ASCs and ASC-derived natural exosomes. In addition, FGF2 levels in the lungs of mice treated with ASC-derived artificial nanovesicles were increased. The uptake of ASC-derived artificial nanovesicles was inhibited by heparin, which is a competitive inhibitor of heparan sulfate proteoglycan that is associated with FGF2 signaling. Taken together, the data indicate that lower doses of ASC-derived artificial nanovesicles may have beneficial effects similar to higher doses of ASCs or ASC-derived natural exosomes in an animal model with emphysema, suggesting that artificial nanovesicles may have economic advantages that warrant future clinical studies.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) represents the third leading cause of death worldwide and affects nearly 5% of the global population.1 In recent decades, new medications have been developed that have dramatically improved the outcomes for patients with COPD; however, these medications target components of the airway disease but not the important emphysema component of COPD.
There are several reports that attribute regenerative effects to stem cells derived from various origins on the emphysema lung in animal models exposed to cigarette smoke, elastase, or VEGFR inhibitor.2, 3, 4 However, only up to 0.01% of stem cells remained in recipient lungs 1 week after a systemic injection.5 In our previous report, results observed using two techniques (fluorescence detection and donor-derived Alu sequence quantification), showed that most ASCs disappeared within 1 day after systemic injection.6 In animal models of lung diseases such as pulmonary fibrosis, COPD, and asthma, the mechanism proposed for stem cell regeneration includes a paracrine route by their soluble mediators that have immunomodulatory, anti-inflammatory and anti-apoptotic effects.2, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15 Our previous report also showed that the conditioned medium from stem cells and stem cells themselves had a similar effect on regeneration from emphysema.3
Exosomes are naturally secreted from cells, and they have a key role in cell-to-cell communication by transferring proteins, mRNAs, and microRNAs.16, 17 Mesenchymal stem cells (MSCs) release exosomes, and there are many studies demonstrating that exosomes from stem cells are candidate secretory mediators. In various disease animal models, including ischemia, acute kidney injury, and acute lung injury, exosomes from stem cells have proven beneficial effects.18, 19, 20, 21 Despite their beneficial effects, the disadvantage of exosomes for clinical applications is that they are naturally released in very small amounts and are biologically heterogeneous.22 Recent reports suggest that nanosized vesicles, called nanovesicles, can be artificially generated from primary cells using a polycarbonate filter.23, 24 Artificially generated nanovesicles from macrophage cell lines target to malignant tumors, and artificial nanovesicles loaded with chemotherapeutics (such as doxorubicin) reduce tumor growth in mice.24 Artificial nanovesicles generated from embryonic stem cells enhance fibroblast proliferation, which is mediated through transforming growth factor-β signaling.23
In our present report, we produced artificial nanovesicles from a subtype of MSCs, namely, adipose-derived stem cells (ASCs). These ASC-derived artificial nanovesicles expressed similar ASC surface markers and growth factors, especially FGF2, compared with ASC-derived natural exosomes. A smaller amount of ASC-derived artificial nanovesicles induced the proliferation of alveolar epithelial cells compared with ASC-derived natural exosomes. The artificial nanovesicles from a lower dose of ASCs showed beneficial effects that were similar to injection of a higher dose of ASCs or ASC-derived natural exosomes in elastase-induced emphysema mice. The uptake of ASCs into alveolar epithelial cells was dependent on heparan sulfate proteoglycan associated with FGF2 signaling.
Materials and methods
Cell culture
ASCs were purchased from Invitrogen (Carlsbad, CA, USA) and cultured using MesenPRO RS medium with supplied supplements (Invitrogen). ASCs were re-fed every 3–4 days with fresh media and were subcultured with 0.05% trypsin-EDTA (Gibco Life Technologies, Grand Island, NY, USA). Artificial nanovesicles were produced on passages 4–5. MLE-12 airway epithelial cells were purchased from the ATCC (Manassas, VA, USA), cultured with the recommended complete growth medium, and subcultured with 0.05% trypsin-EDTA (Gibco Life Technologies).
Production of ASC-derived artificial nanovesicles and natural exosomes
ASCs were resuspended with phosphate-buffered saline (PBS) at a concentration of 5 × 106 ml−1. The resuspended ASCs were sequentially penetrated 10 times through 10 , 5 and 1 μm polycarbonate membranes (Whatman). To remove cellular debris the penetrated mixture was centrifuged with a 10 000 g force for 20 min and filtered using a 0.22-μm syringe filter. To purify artificial nanovesicles from the mixture, the mixture was layered onto 50 and 10% Opti-prep solution (Axid-Shield PoC AS, Dundee, Scotland) and was ultracentrifuged with a 100 000 × g force for 2 h. To prepare exosomes from ASCs, ASCs were washed with PBS twice and incubated overnight with MesenPRO RS medium without supplied supplements. The next day, the medium was collected, filtered with a 0.22-μm filter and ultracentrifuged with a 100 000 g force for 2 h. The pellet after ultracentrifugation was resuspended in PBS and quantified based on a Bradford assay.
Characterization of ASC-derived artificial nanovesicles
The particle size and number of artificial nanovesicles from ASCs was measured using nanoparticle tracking analysis based on light scattering (LM10-HS system, Nanosight Ltd, Wiltshire, UK). Artificial nanovesicles from ASCs were prepared on a copper grid (Spi Supplies, West Chester, PA, USA), and their shape was observed using transmission electron microscopy (TEM, JEM-1011, JEOL Ltd., Tokyo, Japan). Twenty micrograms of artificial nanovesicles from ASCs were loaded onto a gel, resolved by SDS–PAGE and transferred to nitrocellulose membranes, which were blocked in PBS/5% skim milk/0.05% Tween-20. Transferred membranes were incubated at 4 °C overnight with a biotinylated primary antibody including CD63, CD81, CD9, CD73, CD90, CD45, or CD34, incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies, developed with a chemiluminescent membrane substrate (BioFx, Eden Prairie, MN), and exposed to X-ray film (AGFA, Mortsel, Belgium).
Proliferation assay
A Cell Counting Kit-8 (CCK-8) assay (Enzo Life Sciences, New York, USA) was used to measure proliferating cells. The MLE-12 cell line was incubated for 24 h with 0.1 or 1 μg ml−1 of ASC-derived artificial nanovesicles or ASC-derived natural exosomes, and then CCK-8 mixed medium (1:10, CCK-8:medium) was added. The absorbance at 450 nm was measured using a microplate reader (PerkinElmer, Waltham, MA, USA).
Confocal microscopy
Artificial nanovesicles (100 μg) from ASCs were labeled with 1 μM of CellTracker TM CM-DiI (Invitrogen, Life Technologies) for 5 min at 37 °C and then for an additional 15 min at 4 °C. After incubation, the labeled ASC-derived artificial nanovesicles were washed with PBS alternating with ultracentrifugation (100 000 g force, 1 h). MLE-12 cells (3 × 104) were seeded overnight in a 35 mm tissue culture dish or a 27 mm collagen coated tissue culture dish (Nunc). On the next day, cells were pretreated for 30 min with LY294002 (1 μM), cytochalasin D (0.2 μM), chloropromazine (1 μg ml−1), or heparin (10 μg ml−1) (all from Sigma, USA). Then, 10 μg ml−1 of DiI-labeled artificial nanovesicles from ASCs were added to the MLE-12 cells for 2 h, and the cells were counterstained with Hoechst 33258 (Thermo Fisher Scientific). Cells were observed under confocal microscopy (Carl Zeiss, Weimar, Germany), and microscopic images were obtained.
Animal model
C57BL/6 mice were purchased from Orientbio (Seongnam, Korea) and bred in specific pathogen-free facilities at the Asan Medical Center. All experiments using mice were approved by the Institutional Animal Care and Use Committee of the Asan Medical Center (Seoul, Korea). We generated the elastase-induced emphysema model using methods previously described.6 Briefly, mice were intratracheally injected with porcine pancreatic elastase (PPE, Sigma-Aldrich, St. Louis, MO, USA) on day 0, and ASC-derived artificial nanovesicles, ASCs or ASC-derived natural exosomes were intratracheally injected on day 7. On day 14, the mice were euthanized, and their lung tissue was collected.
Ex vivo distribution of artificial nanovesicles
Elastase-induced emphysema mice were intratracheally injected with 25 μg of DiI-labeled artificial nanovesicles from ASCs and were euthanized after 4, 24, 72, or 168 h. Images of the lung were captured using the IVIS Imaging System (PerkinElmer).
Histology and quantification of emphysema
The histology of the lungs was obtained using a method previously described.3 Briefly, the perfused lungs were inflated with 0.5% low-melting agarose, fixed with 4% formalin, and embedded in paraffin. Lung sections of 6-μm thickness were stained with hematoxylin and eosin. The mean linear intercepts were determined using the microscopic images.
FGF2, HGF and VEGF measurement
The levels of FGF2, HGF and VEGF in 2 μg of cell lysates and 2.5 or 10 μg of protein from lung tissue were evaluated using an ELISA. HGF, FGF2 and VEGF levels in the protein lysates were measured by ELISA according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
The data are presented as the mean±s.e. GraphPad Prism5 was used for statistical analysis. The Mann–Whitney U-test was used to compare results between groups. A P-value <0.05 was considered significant. To test for trends, an analysis of variance linearity test was used.
Results
The characterization of artificial nanovesicles from ASCs
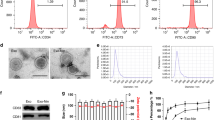
We generated artificial nanovesicles from ASCs using a mini-extruder to pass ASCs through membranes with a series of pore sizes. From 7 × 107 ASCs, we obtained 1 mg of artificial nanovesicles, as measured by the amount of protein inside the nanovesicles. According to TEM images ASC-derived artificial nanovesicles were spherical in shape (Figure 1a). The particle size and number of ASC-derived artificial nanovesicles was measured using nanoparticle tracking analysis. The size of artificial nanovesicles from ASCs was ~100 nm, similar to ASC-derived exosomes (Figure 1b). Approximately 30 × 106 artificial nanovesicle particles were generated from 7 × 107 ASCs, which is ~30 times higher than that of natural exosomes released naturally from ASCs (Figure 1b). ASC-derived artificial nanovesicles were expressed with exosomal markers CD63, CD81 and CD9 (Figure 1c). Positive markers for ASCs, CD73 and CD90, were expressed, and negative markers of ASCs, CD45 or CD34, were not expressed on ASC-derived artificial nanovesicles (Figure 1d). We also measured the amount of certain growth factors related to lung regeneration, such as FGF2, VEGF and HGF, in artificial nanovesicles or natural exosomes from ASCs. Only FGF2 was found to be contained in ASC-derived artificial nanovesicles, and natural exosomes and ASC-derived artificial nanovesicles had a higher amount of FGF2 than ASC-derived natural exosomes (Figure 1e).
Characterization of artificial nanovesicles generated from ASCs. (a) Transmission electron microscopy (TEM) image. (b) Nanoparticle size and number measured by nanoparticle tracking analysis. (c) Western blot analysis of artificial nanovesicles from ASCs using an exosomal marker. (d) Western blot analysis of artificial nanovesicles from ASCs using an ASC marker. NV: ASC-derived artificial nanovesicles, ASC: Adipose-derived stem cells. (e) ELISA measurements of the VEGF, FGF2 and HGF levels in ASC-derived natural exosomes and artificial nanovesicles.
Increased proliferation capacity of an alveolar epithelial cell line induced by artificial nanovesicles from ASCs
Alveolar type II (ATII) cells may be important to lung regeneration from emphysema because they have been known to have a capacity to differentiate into alveolar type I (ATI) cells. Therefore, we observed the effects of ASC-derived artificial nanovesicles on a mouse alveolar epithelial cell line (MLE12 cells). To compare the proliferative capacity between ASC-derived artificial nanovesicles and natural exosomes, MLE12 cells were treated overnight with 0.1 or 1 μg ml−1 of ASC-derived artificial nanovesicles or natural exosomes, respectively. The proliferative capacity was analyzed using a CCK8 assay. ASC-derived artificial nanovesicles had more proliferative capacity compared with ASC-derived natural exosomes at a lower dose (0.1 μg ml−1; Figure 2). The proliferative capacity of ASC-derived artificial nanovesicles in MLE12 cells was dependent on the dose (Supplementary Figure 1a). The wound closure ability of ASC-derived nanovesicles in these cells was also increased (Supplementary Figure 1b). To confirm the effects of FGF2 from ASC-derived artificial nanovesicles in MLE12 cells, we performed mRNA expression analysis using quantitative PCR with a mouse Fgf2-specific primer. The mRNA expression of mouse Fgf2 was increased in MLE12 cells stimulated with the artificial nanovesicles from ASCs (Supplementary Figure 1c). Taken together, our data showed that ASC-derived artificial nanovesicles induced proliferation, wound closure ability and growth factor signaling in MLE12 cells.
Proliferative capacity of ASC-derived natural exosomes and artificial nanovesicles in alveolar epithelial cells. A CCK8 assay was used to evaluate the proliferation rate in the MLE12 cell line incubated with 0.1 or 1 μg ml−1 of ASC-derived natural exosomes and artificial nanovesicles for 24 h. The data were significantly different compared with NoTx (untreated cells) (*P<0.05).
The regenerative effects of ASC-derived artificial nanovesicles in an elastase-induced emphysema mouse model
To compare the regenerative effects among the three groups, ASCs, ASC-derived natural exosomes or ASC-derived artificial nanovesicles, mice were intratracheally injected with 1 × 105 ASCs (1 ×), 1 × or 1/3 × ASC-derived artificial nanovesicles or 1 × ASC-derived natural exosomes (based on protein amounts). Figure 3a shows the histological changes in hematoxylin and eosin stained lung tissue. Quantitative analysis using a mean linear intercept approach is also represented in Figure 3b. Similar regenerative effects were observed in the ASC, 1 × and 1/3 × ASC-derived artificial nanovesicle treated groups. However, 1 × ASC-derived natural exosomes did not have regenerative capacity. Taken together, our findings indicate that ASC-derived artificial nanovesicles may have a more efficient regenerative ability in elastase-induced emphysema lungs because lower doses are equally effective in comparison with ASCs.
Regeneration effects of ASC-derived artificial nanovesicles on elastase-induced emphysema mice. C57BL/6 mice were intratracheally injected with elastase on day 0 and then intratracheally injected with ASC-derived artificial nanovesicles (Nano), natural exosomes (Exo) or ASCs on day 7. (a) Lung histology on day 14 after staining with hematoxylin and eosin. ((−) control: no elastase injection; Ela: 0.4 U of elastase injection; Ela+ASC: elastase+1 × 105 ASCs; Ela+Nano (1/3 ×): elastase+1/3 × artificial nanovesicles; Ela+Nano (1 ×): elastase+1 × artificial nanovesicles; Ela+Exo (1 ×): elastase+1 × exosomes). (b) Morphometric analysis of the mean linear intercept (n=8–12). The data were significantly different (*P<0.05, **P=0.0089, ***P=0.0001) between the elastase group and the groups with injected ASCs or artificial nanovesicles.
Artificial nanovesicles stimulate FGF2 signaling in recipient lungs
In previous reports, the mechanisms of action of ASCs in emphysema animal models were explained by the observation that ASCs could stimulate growth factor signaling, anti-protease activity, and cell survival/proliferation in recipient animals. We therefore compared the mechanisms of action of ASC-derived artificial nanovesicles with those of ASCs and ASC-derived natural exosomes. The level of FGF2 in lung lysates was increased in the mouse emphysema lung after treatment with either ASCs or artificial nanovesicles (1/3 ×) versus the controls (Figure 4a). The levels of VEGF and HGF were not different in lung lysates in all groups (Figure 4b). These results suggest that ASC-derived artificial nanovesicles may stimulate FGF2 signaling to a greater degree than primary ASCs in recipient lungs.
ASCs-derived artificial nanovesicles enhance growth factor expression in recipient lungs. C57BL/6 mice were intratracheally injected with elastase on day 0 and then intratracheally or intravenously injected with 1/3 × of artificial nanovesicles (Nano), 1 × of exosome (Exo) or 1 × 105 of ASCs on day 7. On day 14, mice were sacrificed, and the lungs were collected. ELISA were performed for mouse FGF2 (a), VEGF (b) and HGF (c) using lung protein lysate. (*P<0.05)
Fate of artificial nanovesicles after intratracheal injection into mice
We evaluated the fate of ASC-derived artificial nanovesicles when they were intratracheally injected into elastase-induced emphysema mice. DiI-labeled ASC-derived artificial nanovesicles were intratracheally injected into mice with elastase-induced emphysema, and the fluorescence level in the lung was observed using an optical imaging system at 4, 24, 72 and 168 h after injection. Although we did not conduct detailed observations at a cellular level, we found that ASC-derived artificial nanovesicles remained in the lung for up to 72 h after injection (Figure 5).
Fate of ASC-derived artificial nanovesicles after intratracheal injection into mice. Fluorescence images in the lung at 4, 24, 72 and 168 h after DiI-labeled artificial nanovesicles were intratracheally injected into mice with elastase-induced emphysema. Representative images are shown (n=2) ((−): No nanovesicle injection).
Internalized mechanisms of artificial nanovesicles
We evaluated the internalized mechanisms of ASC-derived artificial nanovesicles using drugs such as LY294002, chlorpromazine, cytochalasin D and heparin to inhibit macropinocytosis, endocytosis, phagocytosis and receptor-dependent endocytosis, respectively. MLE12 cells were treated with each drug for 30 min, and then DiI-labeled-ASC-derived artificial nanovesicles were added. Cells were incubated with the nanovesicles for 2 h and observed with confocal microscopy (Figure 6a). Heparin blocked internalization of ASC-derived artificial nanovesicles in MLE12 cells (Figure 6b). These results suggest that ASC-derived artificial nanovesicles may be internalized and stimulated by receptor-mediated endocytosis.
Internalization mechanisms of ASC-derived artificial nanovesicles. (a) Confocal microscopy of MLE12 cells incubated with/without a 10 μg ml−1 dose of ASC-derived artificial nanovesicles from ASCs for 2 h on a 35 mm tissue culture dish. ((−) Ctrl: without artificial nanovesicles, (+) Ctrl: with nanovesicles, LY294002 (1 μM), cytochalasin D (0.2 μM), chloropromazine (1 μg ml−1), or heparin (10 μg ml−1): cells were treated with each drug for 30 min before artificial nanovesicle treatment). (b) Confocal microscopy of MLE12 cells incubated with a 10 μg ml−1 dose of ASC-derived artificial nanovesicles from ASCs for 2 h on a 27 mm collagen coated tissue culture dish ((+) Ctrl: with nanovesicles, Heparin: heparin (10 μg ml−1) treatment for 30 min before artificial nanovesicle treatment).
Discussion
In our present study, we found that nanovesicles artificially generated from ASCs expressed protein markers of both natural exosomes and stem cells. We could generate artificial nanovesicles at a 30-fold higher level than the number of natural exosomes released naturally from ASCs. ASC-derived artificial nanovesicles contained more FGF2 than ASC-derived exosomes. FGF2 is important in lung development and has regenerative capacity. The proliferative capacity of alveolar epithelial cells was induced by lower doses of ASC-derived artificial nanovesicles compared with ASC-derived natural exosomes. The lower doses of artificial nanovesicles had regenerative effects similar to 105 ASCs (1 ×) or ASC-derived exosomes. The production of FGF2 was increased in the emphysema lung of nanovesicle-treated mice compared with mice treated with ASCs or ASC-derived natural exosomes. The internalization of ASC-derived artificial nanovesicles was inhibited by heparin, which is a competitive inhibitor of cell surface receptor-dependent endocytosis. These results suggest that the more effective regenerative capacity of artificial nanovesicles may be mediated by FGF2 signaling in the nanovesicle-treated mice.
There are some reports that artificial nanovesicles possess biologic activities. Nanovesicles derived from a macrophage cell line are sometimes loaded with doxorubicin, a well-known chemotherapeutic drug. Macrophage-derived nanovesicles can target a tumor site because they express LFA-1, which inhibits the receptors of endothelial CAMs that are associated with excessive angiogenesis for tumor growth. Nanovesicles loaded with doxorubicin have similar effects to 20-fold higher doses of free doxorubicin without showing any side effects.24 Nanovesicles derived from embryonic stem cells (ES cells) improve fibroblast proliferation in a similar fashion to natural exosomes from ES cells, and they carry biological signaling molecules from primary cells into the target cells.23 In this report, we generated 100-nm sized artificial nanovesicles expressing the exosomal and stem cell markers typically present on ASCs. We confirmed that these artificial nanovesicles from ASCs were biologically active in emphysema regeneration and retained primary stem cell characteristics.
The alveolus is covered by ATI cells interspersed with ATII cells.25 Previous reports have shown that the regeneration of damaged alveolar epithelium by bleomycin is mediated by the proliferation of ATIIs and their differentiation into ATIs.26, 27, 28 A decrease in the apoptosis of lung resident cells is also related to lung regeneration in a cigarette smoke-induced emphysema animal model. Apoptosis of alveolar epithelial cells, mesenchymal cells, and endothelial cells is observed in lung biopsies from emphysema patients.29, 30, 31 There is some evidence from studies of lung regeneration implicating ATII cells as endogenous stem/progenitor cells in the alveolus.32, 33 In our current study, we first focused on alveolar epithelial cell proliferation in an in vitro system and found that ASC-derived artificial nanovesicles can increase the proliferation of these cells.
In the past, biologists believed that stem cells directly differentiated into resident cells in damaged tissue, but the concept that stem cells regulate the recipient microenvironment through the secretion of growth factors and immunomodulatory factors is now generally accepted.34 After systemic injection, stem cells disappear within 1 day, but they continue to promote the regeneration of the lung.6 Moreover, the regeneration rates of damaged tissues do not correlate with the degree of engraftment or differentiation of MSCs into resident tissue cells.35 We previously reported that MSC-conditioned medium alone was sufficient to promote regeneration of damaged lung tissues.3 In many COPD animal models, secretory mediators from stem cells significantly contribute to their beneficial effects.36 Exosomes are one type of secretory mediators, which contain many biological molecules, lipids, proteins, growth factor receptors, mRNAs, and microRNAs.35, 37 Some reports suggest that growth factor-related mRNAs inside exosomes are related to the regenerative effect of MSCs.38, 39 In an acute lung injury (ALI) animal model, exosomes derived from MSCs have been reported to show a therapeutic effect mediated by KGF signaling.39 Nanovesicles from ES cells have been found to enhance fibroblast proliferation through transforming growth factor -β signaling.23 It has been previously suggested that the very small amounts of exosomes that are naturally released from stem cells make them a good candidate for clinical applications.22 Therefore, we focused on artificial nanovesicles artificially generated from ASCs as a clinically applicable technology and detected similar biological effects at lower doses compared with ASCs. ASC-derived artificial nanovesicles showed similar therapeutic effects in elastase-induced emphysema mice at lower doses (1/3) than ASCs (105 cells), and ASC-derived exosomes did not have therapeutic effects in these mice at the same ASC doses (105 cells). After intratracheal injection, ASC-derived artificial nanovesicles are detectable for 3 days, in contrast to systemically injected ASCs that disappear within 1 day.6 We would like to explore the mechanism by which artificial nanovesicles enter target cells in a future study. Our present results suggest that ASC-derived artificial nanovesicles might have a sustained effect on host cells.
FGF2 is involved in various biological processes including wound healing, angiogenesis, and especially the alveolar developmental process.40, 41 In an IFN-γ-induced emphysema mice model, FGF2 has a protective role, and recombinant FGF2 helps to lessen the severity of the disease.42 A recent report shows that FGF2 might not be sufficient to repair bleomycin-induced fibrosis, but that this growth factor might be essential to repair and maintain the endothelial barrier in bleomycin-induced lung injury in mice.43 In our current report, we analyzed the production of HGF, VEGF and FGF2 in ASC-derived artificial nanovesicles and found that only FGF2 is contained in artificial nanovesicles from ASCs. Artificial nanovesicles from ASCs increase the production of FGF2 in recipient mice. These data suggest that the regenerative effect of ASC-derived artificial nanovesicles is mediated by the stimulation of recipient growth factor signaling via FGF2.
The function of exosomes can be induced by interacting with target cells through association with the target cell’s plasma membrane. There are several processes of internalization, such as clathrin-mediated endocytosis, macropinocytosis, phagocytosis and receptor-mediated endocytosis. The uptake of rat pheochromocytoma PC12 cell-derived exosomes by clathrin-mediated endocytosis and macropinocytosis was blocked with chlorpromazine and LY294002.44 Exosomes from dendritic cells were also taken up by clathrin-mediated endotycosis.45 Phagocytosis was one of the internalization mechanisms of exosomes from monocytes and macrophages.46 Exosomes from SW780 bladder cancer cells were associated with receptor-mediated endocytosis blocked by heparin.47 We confirmed the internalization mechanisms of ASC-derived artificial nanovesicles based on the well-known processes of exosome uptake by using an inhibitor of each process. The uptake of ASC-derived artificial nanovesicles was inhibited by heparin pretreatment in alveolar epithelial cells. Heparin is a competitive inhibitor of the cell surface receptor dependent on a heparan sulfate proteoglycan coreceptor. Previous reports have shown that heparan sulfate proteoglycan s served as coreceptors for the FGF receptor and modulate FGF2 activity by enhancing FGF2 binding with their receptors.48, 49 These data suggest that FGF2 contained in ASC-derived artificial nanovesicles primarily stimulate recipient cells by receptor-mediated endocytosis.
We artificially generated nanovesicles with a 100-nm size that express exosomal and stem cell markers. ASC-derived artificial nanovesicles contained more FGF2 compared with ASC-derived natural exosomes. ASC-derived artificial nanovesicles had regenerative effects similar to ASCs or ASC-derived exosomes at lower doses in an elastase-induced emphysema model. These regenerative effects are mediated via FGF2 production in this mouse model. The uptake of ASC-derived artificial nanovesicles was inhibited by heparin, which is a competitive inhibitor for heparan sulfate proteoglycan associated with FGF2 signaling. Our collective data suggests that artificial nanovesicles from ASCs may have economic advantages and be clinically applicable to emphysema patients.
References
Pauwels RA, Rabe KF . Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364: 613–620.
Schweitzer KS, Johnstone BH, Garrison J, Rush NI, Cooper S, Traktuev DO et al. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med 2011; 183: 215–225.
Huh JW, Kim SY, Lee JH, Lee JS, Van Ta Q, Kim M et al. Bone marrow cells repair cigarette smoke-induced emphysema in rats. Am J Physiol Lung Cell Mol Physiol 2011; 301: L255–L266.
Katsha AM, Ohkouchi S, Xin H, Kanehira M, Sun R, Nukiwa T et al. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther 2011; 19: 196–203.
Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ . Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med 2006; 173: 171–179.
Kim YS, Kim JY, Shin DM, Huh JW, Lee SW, Oh YM . Tracking intravenous adipose-derived mesenchymal stem cells in a model of elastase-induced emphysema. Tuberc Respir Dis (Seoul) 2014; 77: 116–123.
Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol 2009; 175: 303–313.
Aguilar S, Scotton CJ, McNulty K, Nye E, Stamp G, Laurent G et al. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS ONE 2009; 4: e8013.
Lee SH, Jang AS, Kim YE, Cha JY, Kim TH, Jung S et al. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res 2010; 11: 16.
Zhen G, Liu H, Gu N, Zhang H, Xu Y, Zhang Z . Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front Biosci 2008; 13: 3415–3422.
Zhen G, Xue Z, Zhao J, Gu N, Tang Z, Xu Y et al. Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain-induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy 2010; 12: 605–614.
Bonfield TL, Koloze M, Lennon DP, Zuchowski B, Yang SE, Caplan AI . Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol Lung Cell Mol Physiol 2010; 299: L760–L770.
Cho KS, Roh HJ . Immunomodulatory effects of adipose-derived stem cells in airway allergic diseases. Curr Stem Cell Res Ther 2010; 5: 111–115.
Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci USA 2010; 107: 5652–5657.
Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, LeClair L, Poynter ME et al. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells 2011; 29: 1137–1148.
Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ . Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 2006; 20: 1487–1495.
Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 2011; 68: 2667–2688.
Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 2009; 20: 1053–1067.
Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant 2011; 26: 1474–1483.
Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS ONE 2012; 7: e33115.
Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 2007; 110: 2440–2448.
Thery C, Amigorena S, Raposo G, Clayton A . Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006 Chapter 3 Unit 3.22.
Jeong D, Jo W, Yoon J, Kim J, Gianchandani S, Gho YS et al. Nanovesicles engineered from ES cells for enhanced cell proliferation. Biomaterials 2014; 35: 9302–9310.
Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 2013; 7: 7698–7710.
Stripp BR, Reynolds SD . Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc 2008; 5: 328–333.
Aso Y, Yoneda K, Kikkawa Y . Morphologic and biochemical study of pulmonary changes induced by bleomycin in mice. Lab Invest 1976; 35: 558–568.
Adamson IY . Pulmonary toxicity of bleomycin. Environ Health Perspect 1976; 16: 119–126.
Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA 2011; 108: E1475–E1483.
Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M . Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest 2000; 117: 684–694.
Imai K, Mercer BA, Schulman LL, Sonett JR, D'Armiento JM . Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur Respir J 2005; 25: 250–258.
Yokohori N, Aoshiba K, Nagai A Respiratory Failure Research Group in Japan. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest 2004; 125: 626–632.
Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005; 121: 823–835.
Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 2013; 123: 3025–3036.
Yoshida H, Kitaichi T, Urata M, Kurobe H, Kanbara T, Motoki T et al. Syngeneic bone marrow mononuclear cells improve pulmonary arterial hypertension through vascular endothelial growth factor upregulation. Ann Thorac Surg 2009; 88: 418–424.
Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res 2012; 11: 839–849.
Tzouvelekis A, Ntolios P, Bouros D . Stem cell treatment for chronic lung diseases. Respiration 2013; 85: 179–192.
Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK . Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res 2010; 38: 215–224.
Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev 2013; 22: 772–780.
Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014; 32: 116–125.
Klagsbrun M . The fibroblast growth factor family: structural and biological properties. Prog Growth Factor Res 1989; 1: 207–235.
Martin GR . The roles of FGFs in the early development of vertebrate limbs. Genes Dev 1998; 12: 1571–1586.
Lee BJ, Moon HG, Shin TS, Jeon SG, Lee EY, Gho YS et al. Protective effects of basic fibroblast growth factor in the development of emphysema induced by interferon-gamma. Exp Mol Med 2011; 43: 169–178.
Guzy RD, Stoilov I, Elton TJ, Mecham RP, Ornitz DM . Fibroblast growth factor 2 is required for epithelial recovery, but not for pulmonary fibrosis, in response to bleomycin. Am J Respir Cell Mol Biol 2015; 52: 116–128.
Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem 2014; 289: 22258–22267.
Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 2004; 104: 3257–3266.
Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic 2010; 11: 675–687.
Franzen CA, Simms PE, Van Huis AF, Foreman KE, Kuo PC, Gupta GN . Characterization of uptake and internalization of exosomes by bladder cancer cells. Biomed Res Int 2014; 2014: 619829.
Chua CC, Rahimi N, Forsten-Williams K, Nugent MA . Heparan sulfate proteoglycans function as receptors for fibroblast growth factor-2 activation of extracellular signal-regulated kinases 1 and 2. Circ Res 2004; 94: 316–323.
Esko JD, Selleck SB . Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem 2002; 71: 435–471.
Acknowledgements
We thank the members of the Asan Medical Center animal facility and confocal microscope core for their technical expertise. This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (no. HI14C1487) and research funds from the National Research Foundation of Korea (NRF-2015K1A4A3046807).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Kim, YS., Kim, JY., Cho, R. et al. Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp Mol Med 49, e284 (2017). https://doi.org/10.1038/emm.2016.127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2016.127
This article is cited by
-
Extracellular vesicles and COPD: foe or friend?
Journal of Nanobiotechnology (2023)
-
Bioengineered exosomal-membrane-camouflaged abiotic nanocarriers: neurodegenerative diseases, tissue engineering and regenerative medicine
Military Medical Research (2023)
-
Exosomes derived from adipose-derived stem cells alleviate cigarette smoke-induced lung inflammation and injury by inhibiting alveolar macrophages pyroptosis
Respiratory Research (2022)
-
Artificial exosomes for translational nanomedicine
Journal of Nanobiotechnology (2021)
-
Human umbilical cord mesenchymal stem cell-derived extracellular vesicles ameliorate airway inflammation in a rat model of chronic obstructive pulmonary disease (COPD)
Stem Cell Research & Therapy (2021)