Abstract
When mouse bone marrow-derived macrophages were stimulated with serum amyloid A (SAA), which is a major acute-phase protein, there was strong inhibition of osteoclast formation induced by the receptor activator of nuclear factor kappaB ligand. SAA not only markedly blocked the expression of several osteoclast-associated genes (TNF receptor-associated factor 6 and osteoclast-associated receptor) but also strongly induced the expression of negative regulators (MafB and interferon regulatory factor 8). Moreover, SAA decreased c-fms expression on the cell surface via shedding of the c-fms extracellular domain. SAA also restrained the fusion of osteoclast precursors by blocking intracellular ATP release. This inhibitory response of SAA is not mediated by the well-known SAA receptors (formyl peptide receptor 2, Toll-like receptor 2 (TLR2) or TLR4). These findings provide insight into a novel inhibitory role of SAA in osteoclastogenesis and suggest that SAA is an important endogenous modulator that regulates bone homeostasis.
Similar content being viewed by others
Introduction
Osteoclasts are giant multinucleated cells that are derived from the monocyte lineage.1 Osteoclasts have a crucial role in bone homeostasis through their bone resorbing activity by adhering tightly to the bone surface through interactions with extracellular matrix proteins.2 Two osteoclastogenic mediators, macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor κB ligand (RANKL), induce osteoclast differentiation.3 Because osteoclasts are the only cells that resorb bone in the body,2 the blockade of osteoclast differentiation by inhibiting the action of RANKL or M-CSF and RANKL can effectively block bone erosion in animal models.4, 5
Serum amyloid A (SAA), which is a major acute-phase protein that is released into the circulation in response to infection or injury, is released from liver cells in response to a pro-inflammatory environment.6 Because SAA induces proinflammatory cytokine and chemokine production in several cell types, such as rheumatoid synoviocytes, intestinal epithelial cells, monocytes and neutrophils, SAA is considered to possess cytokine-like properties with immunomodulatory roles. Previous reports have demonstrated that SAA acts on several cell surface receptors.7, 8, 9 SAA selectively stimulates formyl peptide receptor 2 (FPR2), resulting in Ca2+ mobilization and chemotactic migration.7 SAA was also reported to act on Toll-like receptor 2 (TLR2) to elicit intracellular signaling, resulting in G-CSF expression leading to neutrophilia.8 In addition, SAA also has been reported to act on P2X purinoceptor 7 (P2X7), and activate the NOD-like receptor family, pyrin domain-containing 3 inflammasome.9 However, the roles of SAA on osteoclast differentiation have not been examined. In this study, we investigated whether SAA affects osteoclastogenesis in mouse bone marrow-derived osteoclast precursors. We also examined whether FPR2 or TLR2/4 are involved in SAA-induced osteoclastogenesis and in the signaling pathways involved in this process.
Materials and methods
Materials
Human recombinant SAA, murine recombinant M-CSF, human recombinant M-CSF, and murine RANKL were purchased from Peprotech (Rocky Hill, NJ, USA). Pam3CSK4 was purchased from Invitrogen (San Diego, CA, USA). Lipopolysaccharide (LPS) and oxidized ATP were purchased from Sigma-Aldrich (St. Louis, MO, USA). WRWWW.W (WRW4) was synthesized from AnyGen (Gwangju, Korea). Polymyxin B, pertussis toxin, SB203580 and U0126 were purchased from Calbiochem (San Diego, CA, USA). The APC-Anti-mouse CD115(c-fms) was purchased from eBioscience (San Diego, CA, USA). TAPI-1 was purchased from Enzo Life Sciences (Plymouth Meeting, PA, USA). The phospho-ERK, ERK, phospho-p38 mitogen-activated protein kinase (MAPK), p38 MAPK and β-actin antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA).
Osteoclast differentiation and tartrate-resistant acid phosphatase (TRAP) staining
The mouse bone marrow-derived macrophages (BMDMs; 5 × 103 cells per well of 96 well plate) were differentiated into osteoclasts using M-CSF (30 ng ml−1) and RANKL (100 ng ml−1), as previously described.10 Human monocytes were isolated as previously described.11 The human monocytes were differentiated into macrophages by adding M-CSF (30 ng ml−1) for 3 days. The human macrophages (5 × 103 cells per well) were further differentiated into osteoclasts by adding M-CSF (30 ng ml−1) and RANKL (100 ng ml−1) for 17 days, as previously described.12 The differentiated cells were stained for TRAP using an Acid Phosphatase, Leukocyte (TRAP) Kit (Sigma, San Diego, CA, USA). The TRAP+ multinuclear cells (>3 nuclei) with a value >3 were considered to be osteoclasts.
Cell viability assay
The cell viability assay was conducted using the Cell Counting Kit-8 solution (Dojindo, Rockville, MD, USA), as previously described.13
Reverse transcription–PCR analysis
The sequences of the primer used include: RANK: forward, 5′-AGAAGACGGTGCTGGAGTCT-3′, reverse, 5′-TAGGAGCAGTGAACCAGTCG-3′; TNF receptor-associated factor 6 (TRAF6): forward, 5′-GCCCAGGCTGTTCATAATGT-3′, reverse, 5′-TCGCCCACGTACATACTCTG-3′; osteoclast-associated receptor (OSCAR): forward, 5′-CTGCTGGATACGGATCAGCTCCCCAGA-3′, reverse, 5′-CCAAGGAGCCAGAACCTTCGAAACT-3′; TRAP: forward, 5′-CAGTTGGCAGCAGCCAAGGAGGAC-3′, reverse, 5′-TCCGRGCTCGGCGATGGACCAGA-3′; B lymphocyte-induced maturation protein 1 (Blimp1): forward, 5′-TTCTTGTGTGGTATTGTCGGGACTT-3′, reverse, 5′-TTGGGGACACTCTTTGGGTAGAGTT-3′; c-FOS: forward, 5′-AGAGCGGGAATGGTGAAGAC-3′, reverse, 5′-GCTGCATAGAAGGAACCGGA-3′; nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1): forward, 5′-CCAGTATACCAGCTCTGCCA-3′, reverse, 5′-GTGGGAAGTCAGAAGTGGGT-3′; MafB: forward, 5′-AGTGTGGAGGACCGCTTCTCT-3′, reverse, 5′-CAGAAAGAACTCAGGAGAGGAGG-3′; interferon regulatory factor 8 (IRF-8): forward, 5′-CGTGGAAGACGAGGTTACGCTG-3′, reverse, 5′-GCTGAATGGTGTGTGTCATAGGC-3′; and c-fms: forward, 5′-CCAGAGCCCCCACAGATAA-3′, reverse, 5′-AGCTTGGTGTCTCCACGTTTG-3′. For Reverse transcription–PCR, 35 PCR cycles were performed at 94 °C (denaturation, 30 s), 55–65 °C (annealing, 30 s) and 72 °C (extension, 1 min). The PCR products were electrophoresed on a 1.5% agarose gel and visualized by ethidium bromide.
Western blot assay
The cell extracts were prepared by lysing the cells in a buffer containing 20 mM HEPES (pH 7.2), 10% glycerol, 150 mM NaCl, 1% Triton X-100, 50 mM NaF, 1 mM Na3VO4, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin and 1 mM PMSF. The western blot analysis was conducted as previously described.14
Flow cytometry
The osteoclasts or BMDMs were incubated with an anti-murine CD16/32 antibody to block the Fc receptor-mediated antibody binding for 20 min on ice. The cells were then incubated with an anti-mouse CD115 (c-fms) antibody to stain the cell surface expression of c-fms. A FACSCanto II flow cytometer (BD Bioscience, San Jose, CA, USA) was used to detect the c-fms protein on the cell surface.
Extracellular ATP measurement
To measure the extracellular ATP levels, the mouse BMDMs (5 × 103 cells per well) were seeded in 96-well plates and cultured with M-CSF (30 ng ml−1) and RANKL (100 ng ml−1) in minimum essential medium alpha with 10% fetal bovine serum containing minimum essential medium alpha for 3 days at 37 °C and 5% CO2. The cell culture supernatant was collected after 1 h, and the extracellular ATP concentration was measured as previously described.15
Statistical analysis
The results are expressed as the mean±s.e. for the data obtained from the indicated number of experiments. The statistical analysis was performed using Student’s t-test and analysis of variance.
Results
SAA inhibits osteoclast formation from mouse macrophages
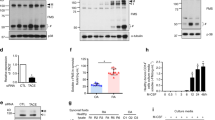
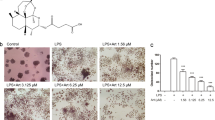
To examine the effects of SAA on osteoclast formation, we stimulated the BMDMs with SAA in the presence of M-CSF and RANKL. Osteoclast formation was induced by incubating the BMDMs with M-CSF plus RANKL, which was identified by TRAP staining (Figure 1a). Under these conditions, the addition of SAA (1 μM) completely inhibited RANKL-induced osteoclast formation (Figure 1a). The SAA-induced inhibitory effect on osteoclast formation was concentration dependent and showed significant effects starting at the 10 nM concentration (Figure 1b). The addition of 100 nM–1 μM SAA almost completely inhibited osteoclast formation (Figure 1b). Because the SAA that we used is a recombinant protein expressed from Escherichia coli, we pretreated cells with an LPS inhibitor (polymyxin B) prior to SAA addition to test for the possible involvement of LPS contamination. Polymyxin B pretreatment did not affect the SAA-induced inhibitory effects on osteoclast formation (Figure 1c). As a control, LPS completely inhibited osteoclast formation, which was markedly reversed by polymyxin B. These results indicate that the inhibitory effects of SAA on osteoclast formation are not a side effect of LPS contamination.
SAA blocks RANKL-induced osteoclastogenesis. (a) Mouse BMDMs were stimulated with SAA (1 μM) in the presence of M-CSF (30 ng ml−1) and RANKL (100 ng ml−1) for 4 days. (b) Mouse BMDMs were stimulated with various concentrations of SAA (0, 1, 10, 100, and 1000 nM) in the presence of M-CSF (30 ng ml−1) and RANKL (100 ng ml−1) for 4 days. (c) Mouse BMDMs were pre-incubated with polymyxin B (10 ng ml−1) prior to SAA (1 μM) and LPS (1 μg ml−1) treatment in the presence of M-CSF (30 ng ml−1) and RANKL (100 ng ml−1) for 4 days. (d) Mouse BMDMs were stimulated with SAA (1 μM) in the presence of M-CSF (30 ng ml−1) and RANKL (100 ng ml−1) for 0, 1, 2, 3 and 4 days. (e) Human monocyte-derived macrophages were stimulated with 1 μM SAA in the presence of M-CSF (30 ng ml−1) and RANKL (100 ng ml−1) for 17 days. After adding the CCK-8 assay solution, the OD was measured at 450 nm (d). All cells were stained using TRAP staining solution. The TRAP+ MNCs (>3 nuclei) were considered to be osteoclasts (a–c, e). The data are expressed as the mean±s.e. of an experiment in duplicate (b–e). The data in the panels are representative of two (e) or three (b–d) independent experiments. *P<0.05 and **P<0.01. BMDM, bone marrow-derived macrophage; M-CSF, macrophage colony-stimulating factor; MNC, multinuclear cell; NS, not significant; NT, not treated; RANKL, receptor activator of nuclear factor κB ligand; SAA, serum amyloid A.
We also determined whether SAA blocks osteoclast formation by inducing cell death in in vitro culture conditions. In the presence of M-CSF, the BMDMs continuously proliferated, whereas the addition of RANKL markedly decreased BMDM proliferation. The BMDMs treated with RANKL and SAA also exhibited increased proliferation compared with the cells treated with RANKL alone (Figure 1d). The results indicate that the inhibitory effects of SAA on osteoclast formation are not caused by cytotoxicity.
To examine whether SAA blocked osteoclast formation in human cells, we tested the effects of SAA on osteoclastogenesis using human macrophages. The incubation of human macrophages with M-CSF and RANKL elicited osteoclast formation as previously reported.12 However, SAA markedly inhibited the RANKL-induced osteoclastogenesis of human macrophages (Figure 1e).
SAA regulates RANKL-induced gene expression
During osteoclast formation, several osteoclast-associated genes, including TRAF6, OSCAR, and NFATc1, are upregulated.16 We also found that the RANKL treatment upregulated the mRNA levels of TRAF6, OSCAR, TRAP, Blimp, c-FOS and NFATc1 (Figure 2a). However, the addition of SAA in the presence of RANKL strongly blocked the upregulation of these osteoclast-associated genes (Figure 2a). RANKL induces cellular signaling by acting on its cell surface receptor, RANK.17 RANKL-induced osteoclast formation is accompanied by increased RANK expression.18 We also observed that RANKL stimulated RANK expression in BMDMs, which was markedly decreased by SAA (Figure 2a). Another TLR2 agonist, Pam3CSK4, also strongly decreased RANKL-induced RANK expression (data not shown). Blimp1 mediates osteoclast formation by blocking the expression of Bcl6, which inhibits the expression of osteoclast-associated gene products such as NFATc1.19 In this study, we also observed that the addition of RANKL induced Blimp1 upregulation. However, the addition of SAA in the presence of RANKL strongly blocked Blimp1 upregulation (Figure 2a). The protein levels of the genes that were downregulated by SAA, such as RANK and TRAF6, were also markedly decreased (Figure 2b).
SAA regulates RANKL-induced gene expression during osteoclastogenesis. (a–c) Mouse BMDMs were stimulated with SAA (1 μM) in the presence of M-CSF (30 ng ml−1) and RANKL (100 ng ml−1) for 3 days. (a, c) The cells were harvested to prepare the RNA. RT-PCR was performed using specific primers for RANK, TRAF6, OSCAR, TRAP, Blimp1, c-FOS, NFATc1, MafB, IRF-8 and GAPDH. (b) The cells were harvested, and Western blot analysis was conducted using anti-RANK, anti-TRAF6 and anti-β-actin antibodies. The results shown are representative of three independent experiments (a–c). BMDM, bone marrow-derived macrophage; M-CSF, macrophage colony-stimulating factor; NT, not treated; RANKL, receptor activator of nuclear factor κB ligand; RT-PCR, reverse transcription–PCR; SAA, serum amyloid A.
Recently, some negative regulators, including MafB and IRF-8, that mediate the inhibition of osteoclast formation have been reported. RANKL downregulates these negative regulators.20, 21 We also showed that the stimulation of BMDMs with RANKL downregulated MafB and IRF-8 (Figure 2c). The addition of SAA markedly reversed the reduced expression of MafB and IRF-8 (Figure 2c).
SAA decreases c-fms expression via ectodomain shedding by tumor necrosis factor-α converting enzyme
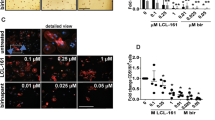
Along with RANK, c-fms, a receptor for M-CSF, is known to be crucial for osteoclast formation.22 Osteoclast-like multinuclear cell formation was reported to be blocked by co-culture with anti-M-CSF or anti-c-fms antibodies.23 Moreover, RANK expression is dependent on M-CSF signaling via c-fms.24 In this study, we investigated the role of SAA in c-fms expression during osteoclast formation. When SAA was added in the presence of RANKL, the levels of the cell surface c-fms protein were dramatically decreased compared with the RANKL-treated group (Figure 3a). The expression of c-fms can be rapidly reduced by shedding of the extracellular domain, and this proteolytic cleavage is mediated by tumor necrosis factor-α converting enzyme (TACE), which is also known as a disintegrin and metalloproteinase 17 (ADAM17).25 TLR ligand-mediated downregulation of c-fms is consistently blocked by a TACE inhibitor.26 Surprisingly, the c-fms expression levels that were downregulated from 82.6 to 1.75% within 1 h were recovered to 78.2% by TAPI-1 (TACE inhibitor) treatment (Figure 3a). TACE-mediated ectodomain shedding is known to be activated by ERK and p38 MAPK.27, 28 Previously, SAA was reported to stimulate ERK and p38 MAPK phosphorylation.29 We tested whether SAA stimulated the activation of ERK and p38 MAPK during osteoclastogenesis by measuring the SAA-induced ERK and p38 MAPK phosphorylation in the presence or absence of RANKL. RANKL is known to induce ERK and p38 MAPK phosphorylation during osteoclast formation.1 We also observed that the stimulation of BMDMs with RANKL elicited ERK and p38 MAPK phosphorylation (Figure 3b). Moreover, SAA further stimulated ERK and p38 MAPK phosphorylation in the presence of RANKL (Figure 3b). To understand the role of ERK and p38 MAPK in SAA-induced c-fms shedding, we measured the surface expression levels of c-fms in the presence of U0126 (a MEK inhibitor) or SB203580 (a p38 MAPK inhibitor). Neither U0126 nor SB203580 affect the SAA-induced reduction in c-fms expression (Figure 3c). However, treatment with both U0126 and SB203580 markedly reversed the SAA-induced downregulation of c-fms expression (Figure 3c). The results suggest that the two MAPK (ERK and p38 MAPK) activities are both required for the downregulation of c-fms by SAA.
SAA stimulates c-fms shedding by TACE, which is dependent on ERK and p38 MAPK activity. (a) Mouse BMDMs were pre-incubated with TAPI-1 (20 μM) for 1 h prior to the SAA (1 μM) treatment. The c-fms levels on the cell surface were determined by flow cytometry using an anti-c-fms antibody. (b) Mouse BMDMs were stimulated with SAA (1 μM) in the presence of M-CSF (30 ng ml−1), RANKL (100 ng ml−1) or M-CSF (30 ng ml−1) and RANKL (100 ng ml−1) for 0, 2, 5, 10, 30 and 60 min. The phosphorylated ERK and p38 MAPK levels were determined by immunoblotting using anti-phospho-ERK and anti-phospho-p38 MAPK antibodies. (c) Mouse BMDMs were pre-incubated for 1 h with U0126 (40 μM; top), SB203580 (20 μM; middle), or U0126 (40 μM) and SB203580 (20 μM; bottom) prior to the SAA (1 μM) treatment. The c-fms levels on the cell surface were determined by flow cytometry using an anti-c-fms antibody. The results shown are representative of three independent experiments (a–c). BMDM, bone marrow-derived macrophage; M-CSF, macrophage colony-stimulating factor; NT, not treated; RANKL, receptor activator of nuclear factor κB ligand; SAA, serum amyloid A.
FPR2, TLR2 and TLR4 are not involved in the SAA-induced inhibitory effects on osteoclast formation
Several cell surface receptors, including FPR2, TLR2 and TLR4, have been reported to act as target molecules for SAA action.20, 21, 30 To test whether FPR2 has a role in the SAA-induced inhibitory effects on osteoclast formation, we first preincubated the BMDMs with an FPR2-selective antagonist, WRW4,31 prior to SAA addition. The inhibitory effects of SAA on osteoclast formation were not reversed by WRW4 (Figure 4a). FPR2 activation induces cellular signaling through pertussis toxin-sensitive G-protein(s).32 The preincubation with pertussis toxin prior to SAA addition in the presence of RANKL did not affect the inhibitory effects of SAA on RANKL-induced osteoclastogenesis in the BMDMs (Figure 4b). These results indicate that FPR2 does not mediate the SAA-induced inhibition of RANKL-induced osteoclastogenesis. We next tested whether TLR2 or TLR4 has a role. First, we confirmed that SAA, LPS (TLR4 agonist) and Pam3CSK4 (TLR2 agonist) completely inhibited osteoclast formation. The Pam3CSK-induced inhibitory effect on osteoclast formation was reversed in the BMDMs derived from the TLR2-deficient mice (Figure 4c). However, the SAA-induced inhibitory effect was not reversed upon TLR2-deficiency (Figure 4c). In the BMDMs isolated from the TLR4 mutant mice (C3H/HeJ), the inhibitory effect on osteoclast formation was reversed by LPS, but not by SAA (Figure 4d). The results indicate that the SAA-induced inhibitory effect on osteoclast formation is not mediated by FPR2, TLR2 or TLR4.
The inhibitory effects of SAA on RANKL-induced osteoclast formation are independent of FPR2, TLR2 or TLR4. (a, b) Mouse BMDMs were pre-incubated with WRW4 (60 μM) or PTX (100 ng ml−1) prior to the SAA (1 μM) treatment in the presence of M-CSF (30 ng ml−1) and RANKL (100 ng ml−1) for 4 days. (c, d) BMDMs were isolated from WT (C57BL/6), TLR2 KO (C57BL/6 background), TLR4 WT (C3H/HeN), and TLR4 mutant (C3H/HeJ) mice. The cells were stimulated with SAA (1 μM), Pam3CSK4 (1 μg ml−1), or LPS (1 μg ml−1) in the presence of M-CSF (30 ng ml−1) and RANKL (100 ng ml−1) for 4 days. (a–d) All cells were stained using the TRAP staining solution. The TRAP+ MNCs (>3 nuclei) were considered to be osteoclasts. The data are expressed as the mean±s.e. of three independent experiments performed in duplicate. *P<0.05 and ***P<0.001. BMDM, bone marrow-derived macrophage; M-CSF, macrophage colony-stimulating factor; MNC, multinuclear cell; NS, not significant; NT, not treated; RANKL, receptor activator of nuclear factor κB ligand; SAA, serum amyloid A.
SAA inhibits RANKL-induced ATP release and the expression of RANKL-induced fusogenic genes
RANKL-induced osteoclast formation was recently reported to be mediated by the activation of the adenosine receptor, which is induced by the generation of adenosine from ATP through apyrase and hexokinase activity.33 The extracellular ATP level can be increased by opening the P2X7 receptor on the cell surface.33 We also found that RANKL-induced osteoclast formation was almost completely inhibited by the addition of oxidized ATP, a P2X7 antagonist (data not shown), supporting the idea that RANKL-induced osteoclast formation is mediated by ATP release. We also observed that the stimulation of osteoclast precursor cells with M-CSF and RANKL strongly increased the extracellular ATP levels (Figure 5a). SAA addition almost completely inhibited the RANKL-induced ATP release (Figure 5a).
SAA decreases the RANKL-induced intracellular ATP release and fusogenic gene expression during osteoclastogenesis. (a) Mouse BMDMs were stimulated with SAA (1 μM) and oATP (200 μM) in the presence of M-CSF (30 ng ml−1) and RANKL (100 ng ml−1) for 3 days. The extracellular ATP concentrations were determined by a bioluminescence assay. (b) Mouse BMDMs were stimulated with SAA (1 μM) in the presence of M-CSF (30 ng ml−1) and RANKL (100 ng ml−1) for 3 days. The cells were harvested to prepare the RNA. RT-PCR was performed using specific primers for DC-STAMP, OC-STAMP, Atp6v0d2 and GAPDH. The results shown are representative of three independent experiments. The data are expressed as the mean±s.e. of an experiment in duplicate (a). The data in the panel are representative of three independent experiments (a). *P<0.05 and **P<0.01. BMDM, bone marrow-derived macrophage; M-CSF, macrophage colony-stimulating factor; NS, not significant; NT, not treated; oATP, oxidized ATP; RANKL, receptor activator of nuclear factor κB ligand; SAA, serum amyloid A.
For the proper formation of functional osteoclasts, osteoclast precursors need to fuse together to generate immature and mature osteoclasts.34 Several genes, such as dendritic cell-specific transmembrane protein (DC-STAMP), osteoclast stimulatory transmembrane protein (OC-STAMP) and Atp6v0d2, have been reported to regulate the fusion process during osteoclast formation.34, 35, 36 We also found that the stimulation of osteoclast precursors with M-CSF and RANKL strongly increased the mRNA levels of DC-DTAMP, OC-STAMP and Atp6v0d2. The addition of SAA markedly reduced the levels of these RANKL-induced osteoclast fusion-associated genes (Figure 5b). The results suggest that SAA may inhibit cell fusion by downregulating the fusogenic genes.
Discussion
Because osteoclasts are the only cells that mediate bone resorption and RANKL induces osteoclast differentiation, the identification of endogenous molecules that inhibit osteoclast differentiation by blocking the action of RANKL is of interest. In this study, we demonstrate that SAA, an endogenous acute phase reactant, strongly inhibits RANKL-induced osteoclast formation, suggesting a novel functional role for SAA in osteoclastogenesis. In this study, we also showed that SAA strongly blocked the fusion of osteoclasts (Figure 5). Previously, LPS has been reported to inhibit osteoclastogenesis from freshly isolated osteoclast precursors, but it was shown to stimulate osteoclast formation from RANKL-primed osteoclast precursors.37 We also tested the effect of SAA at the different time points of osteoclast differentiation and found that SAA strongly blocked osteoclast formation and fusion at the different time points (Figures 1 and 5 and data not shown).
We demonstrate that SAA strongly inhibits RANKL-induced osteoclast formation in a concentration-dependent manner, showing maximal activity at ~1 μM (Figure 1b). We demonstrate that neither of the cell surface receptors FPR2 or TLR2/TLR4 have a role in this inhibition (Figure 4). Both an FPR2 antagonist and a Gi-protein inhibitor, pertussis toxin, failed to inhibit the inhibitory effects of SAA on osteoclast formation (Figure 4). We were also able to rule out the involvement of TLR2 or TLR4 using TLR2-deficient mice or TLR4 mutant mice (Figure 4). Because other receptors, such as CD36 and P2X7, have been reported to mediate the SAA-induced cellular signaling and responses,9, 29 we attempted to examine whether these two receptors were involved in the inhibitory effect of SAA on osteoclast formation. Unfortunately, we could not test the involvement of these receptors because both CD36 and P2X7 are required for RANKL-induced osteoclastogenesis. The inhibition of P2X7 with oxidized ATP completely blocked RANKL-induced osteoclastogenesis (data not shown). The information regarding specific receptor(s) that mediate the inhibitory effect of SAA on RANKL-induced osteoclast formation should be clarified in future work.
Regarding the mechanism of the inhibitory effect of SAA RANKL-induced osteoclastogenesis, we found that SAA blocked osteoclast formation by modulating RANKL-induced gene expression (Figure 2). Fusogenic gene expression in osteoclasts was also markedly decreased by SAA (Figure 5b). These findings suggest that SAA may block the RANKL-RANK-induced signaling pathway that leads to osteoclast formation. The activity of c-fms, an M-CSF receptor, is also crucially required for the proper generation of osteoclasts from precursor cells by RANKL.24 Previously, the TLR ligand-mediated downregulation of c-fms from the cell surface was reported to inhibit osteoclastogenesis.26 Here, we also observed that SAA induced the shedding of the extracellular domain of c-fms (Figure 3a). This TACE-mediated proteolytic cleavage of the c-fms ectodomain was dependent on ERK and p38 MAPK activation (Figure 3c). These results provide a plausible explanation for the high levels of ERK and p38 MAPK phosphorylation induced by SAA in the presence of RANKL, which had been difficult to reconcile with the fact that RANKL-induced ERK and p38 MAPK phosphorylation are required for osteoclast differentiation.1 Our results suggest that SAA may block c-fms signaling by activating ERK and p38 MAPK to stimulate c-fms ectodomain shedding, thus inhibiting osteoclast formation.
During the pathological process of rheumatoid arthritis, SAA levels were reported to be increased in the synovial fluid and SAA was reported to recruit inflammatory cells into the synovium, leading to increased inflammation in the area.38 However, we demonstrate that SAA blocks osteoclast differentiation from BMDMs. This finding suggests that the increased SAA levels in the synovial fluid may promote negative feedback activity in terms of bone homeostasis. The increased SAA levels in the synovial fluid may block osteoclast formation, ultimately resulting in the inhibition of bone resorption. Although SAA has been reported to mediate a proinflammatory response by producing proinflammatory cytokines, a previous report demonstrated that SAA inhibits fever and interleukin-1β- or tumor necrosis factor-α-induced prostaglandin E2 production in vivo.39 The report supports the idea for a possible feedback relationship between SAA and inflammatory cytokines such as tumor necrosis factor-α and RANKL.
References
Boyle WJ, Simonet WS, Lacey DL . Osteoclast differentiation and activation. Nature 2003; 423: 337–342.
Teitelbaum SL . Bone resorption by osteoclasts. Science 2000; 289: 1504–1508.
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 1998; 95: 3597–3602.
Kitaura H, Zhou P, Kim HJ, Novack DV, Ross FP, Teitelbaum SL . M-CSF mediates TNF-induced inflammatory osteolysis. J Clin Invest 2005; 115: 3418–3427.
Pettit AR, Ji H, von Stechow D, Müller R, Goldring SR, Choi Y et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol 2001; 159: 1689–1699.
Furlaneto CJ, Campa A . A novel function of serum amyloid A: a potent stimulus for the release of tumor necrosis factor-α, interleukin-1β, and interleukin-8 by human blood neutrophil. Biochem Biophys Res Commun 2000; 268: 405–408.
Su SB, Gong W, Gao JL, Shen W, Murphy PM, Oppenheim JJ et al. A seven-transmembrane, G protein–coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med 1999; 189: 395–402.
He RL, Zhou J, Hanson CZ, Chen J, Cheng N, Ye RD . Serum amyloid A induces G-CSF expression and neutrophilia via Toll-like receptor 2. Blood 2009; 113: 429–437.
Niemi K, Teirila L, Lappalainen J, Rajamaki K, Baumann MH, Öörni K et al. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol 2011; 186: 6119–6128.
Lee Y, Kim HJ, Park CK, Kim YG, Lee HJ, Kim JY et al. MicroRNA-124 regulates osteoclast differentiation. Bone 2013; 56: 383–389.
Bae YS, Bae H, Kim Y, Lee TG, Suh PG, Ryu SH . Identification of novel chemoattractant peptides for human leukocytes. Blood 2001; 97: 2854–2862.
Gupta N, Barhanpurkar AP, Tomar GB, Srivastava RK, Kour S, Pote ST et al. IL-3 inhibits human osteoclastogenesis and bone resorption through downregulation of c-Fms and diverts the cells to dendritic cell lineage. J Immunol 2010; 185: 2261–2272.
Li N, Sun C, Zhou B, Xing H, Ma D, Chen G et al. Low concentration of quercetin antagonizes the cytotoxic effects of anti-neoplastic drugs in ovarian cancer. PLoS ONE 2014; 9: e100314.
Lee HY, Lee SY, Kim SD, Shim JW, Kim HJ, Jung YS et al. Sphingosylphosphorylcholine stimulates CCL2 production from human umbilical vein endothelial cells. J Immunol 2011; 186: 4347–4353.
Brandao-Burch A, Key ML, Patel JJ, Arnett TR, Orriss IR . The P2X7 Receptor is an Important Regulator of Extracellular ATP Levels. Front Endocrinol (Lausanne) 2012; 3: 41.
Feng X . RANKing intracellular signaling in osteoclasts. IUBMB life 2005; 57: 389–395.
Wada T, Nakashima T, Hiroshi N, Penninger JM . RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med 2006; 12: 17–25.
Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Yano K et al. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun 1998; 253: 395–400.
Miyauchi Y, Ninomiya K, Miyamoto H, Sakamoto A, Iwasaki R, Hoshi H et al. The Blimp1–Bcl6 axis is critical to regulate osteoclast differentiation and bone homeostasis. J Exp Med 2010; 207: 751–762.
Kim K, Kim JH, Lee J, Jin HM, Kook H, Kim KK et al. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood 2007; 109: 3253–3259.
Zhao B, Takami M, Yamada A, Wang X, Koga T, Hu X et al. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat Med 2009; 15: 1066–1071.
Ross FP . M-CSF, c-Fms, and Signaling in Osteoclasts and their Precursors. Ann NY Acad Sci 2006; 1068: 110–116.
Tanaka S, Takahashi N, Udagawa N, Tamura T, Akatsu T, Stanley ER et al. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest 1993; 91: 257–263.
Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor κB (RANK) receptors. J Exp Med 1999; 190: 1741–1754.
Rovida E, Paccagnini A, Del Rosso M, Peschon J, Sbarba PD . TNF-α-converting enzyme cleaves the macrophage colony-stimulating factor receptor in macrophages undergoing activation. J Immunol 2001; 166: 1583–1589.
Ji JD, Park-Min KH, Shen Z, Fajardo RJ, Goldring SR, McHugh KP et al. Inhibition of RANK expression and osteoclastogenesis by TLRs and IFN-γ in human osteoclast precursors. J Immunol 2009; 183: 7223–7233.
Soond SM, Everson B, Riches DW, Murphy G . ERK-mediated phosphorylation of Thr735 in TNFα-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci 2005; 118: 2371–2380.
Xu P, Derynck R . Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol Cell 2010; 37: 551–566.
Baranova IN, Vishnyakova TG, Bocharov AV, Kurlander R, Chen Z, Kimelman ML et al. Serum amyloid A binding to CLA-1 (CD36 and LIMPII analogous-1) mediates serum amyloid A protein-induced activation of ERK1/2 and p38 mitogen-activated protein kinases. J Biol Chem 2005; 280: 8031–8040.
Sandri S, Rodriguez D, Gomes E, Monteiro HP, Russo M, Campa A . Is serum amyloid A an endogenous TLR4 agonist? J Leukoc Biol 2008; 83: 1174–1180.
Bae YS, Lee HY, Jo EJ, Kim JI, Kang HK, Ye RD et al. Identification of peptides that antagonize formyl peptide receptor-like 1-mediated signaling. J Immunol 2004; 173: 607–614.
Migeotte I, Communi D, Parmentier M . Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev 2006; 17: 501–519.
Pellegatti P, Falzoni S, Donvito G, Lemaire I, Di Virgilio F . P2X7 receptor drives osteoclast fusion by increasing the extracellular adenosine concentration. FASEB J 2011; 25: 1264–1274.
Ishii M, Saeki Y . Osteoclast cell fusion: mechanisms and molecules. Mod Rheumatol 2008; 18: 220–227.
Yang M, Birnbaum MJ, MacKay CA, Mason-Savas A, Thompson B, Odgren PR . Osteoclast stimulatory transmembrane protein (OC-STAMP), a novel protein induced by RANKL that promotes osteoclast differentiation. J Cell Physiol 2008; 215: 497–505.
Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N et al. v-ATPase V0 subunit d2–deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med 2006; 12: 1403–1409.
Liu J, Wang S, Zhang P, Said-Al-Naief N, Michalek SM, Feng X . Molecular mechanism of the bifunctional role of lipopolysaccharide in osteoclastogenesis. J Biol Chem 2009; 284: 12512–12523.
O'Hara R, Murphy EP, Whitehead AS, FitzGerald O, Bresnihan B . Acute-phase serum amyloid A production by rheumatoid arthritis synovial tissue. Arthritis Res Ther 2000; 2: 142–144.
Shainkin-Kestenbaum R, Berlyne G, Zimlichman S, Sorin HR, Nyska M, Danon A . Acute Phase Protein, Serum Amyloid A, Inhibits IL-1-and TNF-Induced Fever and Hypothalamic PGE2 in Mice. Scand J Immunol 1991; 34: 179–183.
Acknowledgements
This work was supported by grants from the National Research Foundation of Korea (No. 2012R1A2A2A01007751) and the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI13C1857).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Oh, E., Lee, H., Kim, H. et al. Serum amyloid A inhibits RANKL-induced osteoclast formation. Exp Mol Med 47, e194 (2015). https://doi.org/10.1038/emm.2015.83
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2015.83
This article is cited by
-
Staphylococci planktonic and biofilm environments differentially affect osteoclast formation
Inflammation Research (2023)
-
FAM19A5, a brain-specific chemokine, inhibits RANKL-induced osteoclast formation through formyl peptide receptor 2
Scientific Reports (2017)
-
Inhibition of Osteoclast Differentiation and Bone Resorption by Bisphosphonate-conjugated Gold Nanoparticles
Scientific Reports (2016)