Abstract
Hepatic ischemia/reperfusion (I/R) injury leads to oxidative stress and acute inflammatory responses that cause liver damage and have a considerable impact on the postoperative outcome. Much research has been performed to develop possible protective techniques. We aimed to investigate the efficacy of SPA0355, a synthetic thiourea analog, in an animal model of hepatic I/R injury. Male C57BL/6 mice underwent normothermic partial liver ischemia for 45 min followed by varying periods of reperfusion. The animals were divided into three groups: sham operated, I/R and SPA0355 pretreated. Pretreatment with SPA0355 protected against hepatic I/R injury, as indicated by the decreased levels of serum aminotransferase and reduced parenchymal necrosis and apoptosis. Liver synthetic function was also restored by SPA0355 as reflected by the prolonged prothrombin time. To gain insight into the mechanism involved in this protection, we measured the activity of nuclear factor-κB (NF-κB), which revealed that SPA0355 suppressed the nuclear translocation and DNA binding of NF-κB subunits. Concomitantly, the expression of NF-κB target genes such as IL-1β, IL-6, TNF-α and iNOS was significantly downregulated. Lastly, the liver antioxidant enzymes superoxide dismutase, catalase and glutathione were upregulated by SPA0355 treatment, which correlated with the reduction in serum malondialdehyde. Our results suggest that SPA0355 pretreatment prior to I/R injury could be an effective method to reduce liver damage.
Similar content being viewed by others
Introduction
Ischemia/reperfusion (I/R) injury is a pathophysiologic process in which cellular damage is accentuated following oxygen delivery to the ischemic tissue. Liver I/R injury is a major cause of morbidity and mortality after resection surgery, liver transplantation, and hemorrhagic and septic shock.1 Partial or total interruption of blood flow during liver surgery is called ischemia. During this period, the lack of tissue oxygen results in conversion of cellular metabolism from aerobic to anaerobic pathways and this metabolic change causes various hepatocellular dysfunctions. Upon revascularization, reoxygenation of the ischemic tissue generates various reactive oxygen species (ROS), which further contribute to profound hepatocellular injury—a phenomenon known as reperfusion injury.2 Experimental evidence suggests that Kupffer cells are responsible for ROS generation in the early phase of reperfusion injury (up to 2 h after reperfusion).3, 4 In addition to ROS, Kupffer cells produce and secrete proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1 and IL-6.5 These cytokines in turn attract and activate neutrophils during the late phase (6 h after reperfusion) of reperfusion injury.6 Once neutrophils are recruited into the ischemic area, they further release ROS, cytokines, myeloperoxidase (MPO) and various other mediators, all of which amplify the tissue damage.7, 8
Production of these proinflammatory cytokines/chemokines is regulated by the transcription factor nuclear factor-κB (NF-κB), which is activated upon I/R injury.1, 9 Additionally, NF-κB activation plays critical roles in the regenerative and antiapoptotic responses;9, 10, 11 therefore, inhibition of NF-κB activation could contribute to preventing I/R injury. Indeed, several NF-κB suppressors, NF-κB decoy oligonucleotides and hepatocyte-specific ablation of IκB kinase (IKK) β have been proved to be effective in preventing I/R injury.12, 13, 14, 15
Previously, we synthesized SPA0355, a thiourea analog, and reported its anti-inflammatory effect through suppression of the NF-κB pathway in an animal experimental model of arthritis.16 In our latest publication, we showed that SPA0355 downregulated cytokine-induced pancreatic β-cell apoptosis by blocking the NF-κB and JAK-STAT pathways.17 In the present study, we demonstrate that SPA0355 administration before liver I/R injury exerts hepatoprotective effects in a warm I/R liver injury model.
Materials and methods
Animals
Pathogen-free 8- to 10-week-old C57BL/6 male mice (Orient, Seoul, Korea) were maintained on a standard laboratory chow diet and water ad libitum. All experimental protocols were approved by the Institutional Animal Care and Use Committee at Chonbuk National University.
Preparation of SPA0355
Preparation of SPA0355 (1-Methyl-3-[4-(2-phenoxazin-10-ylethoxy)phenyl]thiourea) was performed as previously described.16
Model of partial hepatic I/R injury
Mice were anesthetized with ketamine (100 mgkg−1) and xylazine (10 mgkg−1) by intraperitoneal injection. A midline incision was performed and an atraumatic clip was placed across the portal vein, hepatic artery and bile duct just above the right branch. This interrupted blood flow to the left lateral and median lobes that represents approximately 70% of the total blood supply to the liver. The liver was kept moist with gauze soaked in saline, and body temperature was maintained at 37 °C with a warm blanket throughout ischemia. After 45 min of partial hepatic ischemia, the clip was removed to initiate reperfusion (Figure 1). Sham mice underwent the same operation without vascular occlusion. After the desired time period of reperfusion, the mice were killed by exsanguination under anesthesia and serum samples were collected. Left lateral and median lobes of the liver were collected and immediately fixed in 10% formalin or stored at −80 °C until further analysis.
Schematic diagram of the experimental protocol. The following assays were performed after the reperfusion: Western blotting and real-time RT–PCR analyses for NF-κB activation (1 h); AST, ALT, ELISA for cytokines and PT assays (6 h); and MPO, MDA, CAT, SOD and GSH assays and western blotting for apoptosis and end-point histology (24 h). ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAT, catalase; MPO, myeloperoxidase; PT, prothrombin time; RT–PCR, real-time RT–PCR; WB, western blotting.
Quantification of liver injury
Liver cell integrity was determined by assaying plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity (Asan Pharm, Seoul, Korea). Hepatocellular function was evaluated by measuring prothrombin time, which was quantified using a portable coagulometer (CoaguChek XS, Roche Diagnostics, Mannheim, Germany).
Protein extraction
Whole cell protein was extracted from liver tissue with T-PER tissue protein extraction reagent (Pierce Biotechnology, Rockford, IL, USA). For nuclear protein extraction, the liver samples were collected 60 min after reperfusion and immediately homogenized with a prechilled Dounce homogenizer (Wheaton Industries, Millville, NJ, USA). Nuclear protein was extracted with NE-PER nuclear and cytoplasmic extraction reagents (Pierce Biotechnology). Protein concentration was determined using the Bradford method.
Western blot analysis
Liver homogenates containing 30 μg of whole cell lysate protein or 10 μg of nuclear protein were separated by 10 or 12% SDS-PAGE and transferred to PVDF membranes. After blocking with 5% skim milk, the blot was probed with primary antibodies for p50, p65, IκBα, IKKα, IKKβ, PCNA and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and cleaved caspase-3, Bcl-2, Bax, p-IKKα and p-IKKβ (Cell Signaling Technology, Beverly, MA, USA). Horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (Santa Cruz Biotechnology) was used as a secondary antibody.
Electrophoretic mobility shift assay
Nuclear extracts prepared from the liver tissues were incubated with a proteinase inhibitor cocktail (Thermo, San Diego, CA, USA) to inhibit endogenous protease activity. Oligonucleotide corresponding NF-κB site (5′-CCGGTTAACAGAGGGGGCTTTCCGAG-3′) was synthesized and used as a probe for a gel retardation assay. The two complementary strands were then annealed and labeled with α-32P-dCTP. Labeled oligonucleotide (10 000 counts per minute), 10 μg of nuclear extract, and binding buffer (10 mM Tris-HCl, pH 7.6, 500 mM KCl, 10 mM EDTA, 50% glycerol, 100 ng poly[dI·dC], 1 mM dithiothreitol) were then incubated for 30 min at room temperature in a final volume of 20 μl. Next, the reaction mixtures were analyzed by electrophoresis on 4% polyacrylamide gels in a 0.5 × Tris-borate buffer. The gels were dried and examined by autoradiography. The specificity of the DNA–protein interaction for NF-κB was confirmed by competition assays using a 50-fold excess of unlabeled oligonucleotide.
Histological study
Fixed liver tissues were embedded in paraffin. Tissue sections (5 μm) were stained with hematoxylin and eosin for light microscopy. TUNEL staining was performed using a commercial kit (R&D Systems, Minneapolis, MN, USA). Five to six random sections were investigated per slide to determine the percentage of necrotic cells.
RNA isolation and real-time RT–PCR
Total RNA was extracted from frozen liver tissue using Trizol reagent (Invitrogen, Carlsbad, CA, USA). First-strand cDNA was generated using the random hexamer primer provided in the first-strand cDNA synthesis kit (Applied Biosystems, Foster City, CA, USA). Specific primers for each gene (Table 1) were designed using primer express software (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an invariant control. The real-time RT–PCR reaction mixture consisted of 10 ng reverse transcribed RNA, 200 nM forward and reverse primers, and 2 × PCR master mixture in a final volume of 10 μl. The PCR reaction was carried out in 384-well plates using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems).
Activities of superoxide dismutase, catalase, glutathione and malonidialdehyde
To analyze enzyme activities of superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH), liver tissues were suspended in 10 mM phosphate buffer (pH 7.4), mixed with ice-cold 5% metaphosphoric acid solution and then homogenized. The homogenates were centrifuged at 3000 × g for 10 min. The enzyme activities in the supernatant were determined using commercial assay kits (Enzo Life Sciences, Inc., Plymouth Meeting, PA, USA).
Malondialdehyde (MDA) concentration in plasma was determined using a kit from Enzo Life Sciences, Inc. The method is based on the reaction of MDA with a chromogenic reagent, N-methyl-2-phenylindole. The absorbance of chromogenic product was measured at 586 nm.
MPO assay
Liver samples were homogenized and the pellet was resuspended in 1% hexadecyltrimethylammonium. After heating for 2 h at 60 °C, samples were centrifuged and the supernatant was incubated with 3,3′,3,5′-tetramethylbenzidine followed by addition of H2O2. The change in optical density was measured at 650 nm.
Statistical analysis
Data were expressed as mean±s.e.m. Statistical analyses were performed using one-way ANOVA for the comparison of data from different treatment groups. Differences with a P value <0.05 were considered statistically significant.
Results
Intraperitoneal administration of SPA0355 attenuates liver injury and maintains liver function after I/R
Liver injury was assessed by measuring serum levels of AST and ALT, and by determining prothrombin time. I/R injury induced significant increases in serum ALT and AST levels compared with sham-operated mice (Figure 2a). However, pre-treatment with SPA0355 administered intraperitoneally three times before I/R injury augmented the elevation of serum ALT and AST levels in a dose-dependent manner. Specifically, 2.5 μgkg−1 SPA0355 reduced the AST level by 19.6% (P<0.05) and the ALT level by 21.3% (P<0.05), and the 5 μgkg−1 dose further reduced AST and ALT levels by 60.8% (P<0.01) and 59.2% (P<0.01), respectively. It is worth noting that no statistical difference was found between 5 and 10 μgkg−1 SPA0355, suggesting that 5 μgkg−1 of SPA0355 is sufficient to achieve full efficacy. In addition, prothrombin time was measured as a marker of liver synthetic function. I/R injury resulted in prolonged prothrombin time at 6 h after reperfusion, which was significantly decreased by pretreatment with 5 μgkg−1 SPA0355 (Figure 2b).
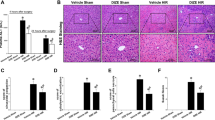
Effect of SPA0355 on I/R-induced hepatocellular injury in mice. Mice were pretreated with the indicated concentrations of SPA0355 intraperitoneally three times before undergoing 45 min of hepatic ischemia. After 6 h reperfusion, serum levels of ALT and AST (a) and prothrombin time (b) were analyzed. (c) After 24 h reperfusion, liver tissues were fixed by 10% formalin, and liver necrosis and apoptosis were assessed by H&E and TUNEL staining, respectively. The area of necrosis was analyzed as described in the Materials and methods and TUNEL-positive apoptotic cells were counted and expressed as a percentage of all hepatocytes. Values are the mean±s.e.m. of three independent experiments (n=9 mice per group). **P<0.01 vs sham-operated mice; #P<0.05, ##P<0.01 vs I/R mice. H&E, hematoxylin and eosin; SPA5, 5 μgkg−1 SPA0355; VEH, vehicle.
Histologic changes following I/R were consistent with biochemical parameters of hepatic injury. Hematoxylin and eosin staining revealed extensive hepatocellular necrosis and sinusoidal congestion in mice subjected to 45 min of ischemia and 24 h of reperfusion compared with the sham group (Figure 2c), with the area of necrosis being significantly different between the two groups. However, in mice that were pretreated with SPA0355, there were considerable areas of normal liver architecture and the area of necrosis was significantly lower compared with the I/R group.
Although the major cause of cell death during hepatic I/R injury is necrosis, apoptotic cell death is also observed during the injury process.18 The number of apoptotic cells in I/R-injured liver tissues was determined by TUNEL staining. As shown in Figure 2c, the number of TUNEL-positive apoptotic cells was significantly higher in the I/R-injured mice compared with that in the sham group. This observation correlated with increased protein levels of proapoptotic Bax and cleaved caspase-3 and decreased protein level of antiapoptotic Bcl-2 (Figure 3). When mice were treated with SPA0355 before I/R injury, a significant reduction in apoptotic cells and concordant changes in apoptotic proteins were observed. Together, these results suggest that SPA0355 has protective effects against hepatic I/R injury.
Effect of SPA0355 on I/R-induced apoptosis. Liver tissues were retrieved 24 h after reperfusion and the expression levels of Bax, cleaved caspase-3 and Bcl-2 were examined by western blotting. Protein intensity was measured. Values are the mean±s.e.m. of three independent experiments (n=9 mice per group). **P<0.01 vs sham-operated mice; ##P<0.01 vs I/R mice.
SPA0355 decreases neutrophil infiltration
MPO, an enzyme that is predominantly stored in azurophilic neutrophil granules, was used to quantify neutrophil infiltration in the liver. After 24 h of reperfusion, MPO activity increased markedly in the I/R-injured mice compared with the sham group (Figure 4). Consistent with the observed decrease in liver damage, the MPO activity of mice that were pretreated with 5 μgkg−1 SPA0355 was 48.5% of that in the vehicle group. These results suggest that suppression of neutrophil infiltration by SPA0355 may further contribute to protection against I/R injury.
SPA0355 inhibits the NF-κB signaling pathway
To determine whether SPA0355 exerted a liver protective effect through suppression of NF-κB activation, we measured protein levels of p65 and p50 subunits in nuclear extracts and DNA binding of NF-κB subunits prepared 1 h after reperfusion. I/R mice showed increases in nuclear p65 and p50 protein levels (Figure 5a) and binding activity of the p65 subunit to an NF-κB consensus sequence (Figure 5b) compared with sham-operated mice. The DNA-bound p65 subunit of NF-κB was resolved by supershift (Figure 5b, lane 5). In contrast, nuclear extracts prepared from mice that were pretreated with SPA0355 revealed markedly suppressed nuclear translocation and DNA binding of NF-κB. Consistently, I/R mice showed decreased levels of IκBα protein in the cytoplasm because of IκBα degradation, as compared with a similar fraction from sham mice, but IκBα degradation was markedly suppressed by SPA0355 pretreatment (Figure 5a). Next, we tested the effect of SPA0355 on IKK activation, which is required for IκB degradation. SPA0355 had no effect on IKKα and IKKβ protein levels, but suppressed I/R-induced IKK activity, as shown by decreased levels of phosphorylated forms of IKKα and IKKβ (Figure 5c). Together, these results suggested that SPA0355 inhibited the IκBα degradation by reducing IKK activities, thereby preventing subsequent NF-κB activation.
Effect of SPA0355 on I/R-induced NF-κB activation. Nuclear and cytoplasmic proteins were extracted from liver homogenates after 1 h of reperfusion and nuclear translocation of p50 and p65 subunits and cytoplasmic IκBα degradation (a), NF-κB DNA binding activity (b), and protein levels and phosphorylation of IKKα and IKKβ (c) were analyzed. After 1 h reperfusion, mRNA levels of IL-1β, IL-6, TNF-α and iNOS were analyzed by real-time RT–PCR (d). Values are the mean±s.e.m. of three independent experiments (n=9 mice per group). **P<0.01 vs sham-operated mice; #P<0.05, ##P<0.01 vs I/R mice.
To further examine the effects of SPA0355 on NF-κB suppression, mRNA levels of NF-κB-dependent inflammatory mediators were measured by real-time RT-PCR. We observed a significant increase in IL-1β, IL-6, TNF-α and iNOS mRNA expression after hepatic I/R injury (Figure 5d). However, prior injection with SPA0355 resulted in a dramatic decrease in expression of all of these mRNAs, indicating that the I/R-induced increase in inflammatory mediator levels could be downregulated by SPA0355 treatment.
SPA0355 suppresses oxidative stress
The serum level of MDA, an indicator of oxidative damage, was also determined. As shown in Figure 6, I/R significantly increased the MDA content in liver tissues compared with the sham group. Consistent with this finding, I/R caused significant suppression of the hepatic antioxidant defense, as observed by a decrease in the glutathione level and in SOD and CAT enzyme activities. Pretreatment with SPA0355 reversed these changes caused by I/R; the serum level of MDA was significantly lower and hepatic levels of glutathione, SOD and CAT were significantly higher in the SPA0355 group than in the I/R group.
Effect of SPA0355 on I/R-induced oxidative stress. The plasma level of MDA and tissue levels of GSH, SOD and CAT were analyzed. Values are the mean±s.e.m. of three independent experiments (n=9 mice per group). **P<0.01 vs sham-operated mice; #P<0.05, ##P<0.01 vs I/R mice. CAT, catalase; GSH, glutathione; MDA, malondialdehyde; SOD, superoxide dismutase.
Discussion
I/R injury is, at least in part, a result of the inflammatory response, and NF-κB activation is central to the acute inflammatory response in I/R-injured liver tissue.1, 9 In this respect, the use of NF-κB inhibitors to limit hepatic I/R injury has attracted attention. In this study, we tested whether SPA0355, which is known to have a NF-κB suppressive effect, would protect against hepatic I/R injury. Indeed, SPA0355 pretreatment in a mouse model of partial hepatic I/R resulted in a significant improvement in the plasma biochemical parameters of hepatocellular injury and amelioration of histopathologic findings of tissue damage in the liver.
As a key regulator of inflammatory and cell survival responses, NF-κB participates in both the generation of proinflammatory mediators and the antiapoptotic pathway, and the balance between these determines the fate of I/R-injured liver tissue.19 These contradictory roles of NF-κB help to explain several conflicting reports that NF-κB inactivation either protects against hepatic I/R injury12, 14, 20 or aggravates such injury21, 22, 23 although the underlying reasons for the conflicting activities are not entirely clear. Because NF-κB is differentially activated according to several environmental factors such as warm or cold I/R, partial or total I/R, species and genetic background of the animal, and duration and intensity of I/R, NF-κB activation should not be thought of as an event that aggravates or protects the liver, but rather is responsible for maintaining liver homeostasis during I/R injury.9, 23 In our experimental conditions, tissue levels of proinflammatory mediators were markedly suppressed and the number of TUNEL-positive apoptotic hepatocytes was significantly reduced by SPA0355 treatment, suggesting that NF-κB suppression by SPA0355 shifted the balance toward suppression of the inflammatory response.
Reperfusion of the ischemic liver activates Kupffer cells, leading to the generation of ROS and expression of proinflammatory cytokines such as TNF-α and IL-β.5 These cytokines upregulate the expression of adhesion molecules and recruit neutrophils into the liver, which leads to further production of ROS.6 Previous studies have clearly demonstrated that ROS generation by either Kupffer cells or infiltrated neutrophils is responsible for tissue injury after reperfusion.8 Accordingly, transgenic mice that overexpress SOD and CAT are protected against hepatic I/R injury.24, 25 Similarly, intravenous administration of GSH also protects hepatocytes from I/R injury and improves animal survival.26 In our study, hepatic I/R injury resulted in a significant increase in tissue MPO activity (an index of neutrophil infiltration), a decrease in tissue levels of SOD, CAT and GSH (indices of antioxidant capacities), and an increase in the plasma level of MDA (an index of lipid peroxidation). However, SPA0355 treatment dramatically reversed these changes, indicating that SPA0355 protected hepatocytes by reducing the oxidative stress caused by I/R injury.
Although necrosis is the major pathologic event during I/R injury, apoptotic cell death is also induced after hepatic I/R injury. Infiltrated neutrophils are known to mediate both apoptotic cell death and inflammation;2 in fact, several pharmacologic or genetic interventions that suppress neutrophil infiltration have been shown to ameliorate both inflammation and apoptotic cell death after hepatic I/R injury.27, 28, 29, 30 Under our experimental conditions, SPA0355 effectively reduced apoptotic damage as evidenced by a significant reduction in the number of TUNEL-positive apoptotic cells and decreased expression of apoptosis-related genes. As discussed earlier, SPA0355 suppressed neutrophil recruitment into the ischemic liver tissue, which might account for the reduction in apoptotic death of hepatocytes.
In summary, we provide evidence that SPA0355 protects against I/R-induced liver damage. The mechanism of action of SPA0355 involves attenuation of NF-κB activation and the subsequent expression of proinflammatory mediators and suppression of oxidative stress, neutrophil infiltration and apoptosis. In both this and our previous studies, SPA0355 was well tolerated at the administered dose with no evidence of drug toxicity.16, 17 Therefore, SPA0355 can be considered as a therapeutic option to prevent liver damage after medical or surgical conditions that interrupt blood flow to liver tissue.
References
Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ . Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology 2000; 32: 169–173.
Jaeschke H . Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol 2011; 26: 173–179.
Jaeschke H, Farhood A . Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol 1991; 260: G355–G362.
Muriel P . Role of free radicals in liver diseases. Hepatol Int 2009; 3: 526–536.
Wanner GA, Ertel W, Muller P, Hofer Y, Leiderer R, Menger MD et al. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock 1996; 5: 34–40.
Shibuya H, Ohkohchi N, Tsukamoto S, Satomi S . Tumor necrosis factor-induced, superoxide-mediated neutrophil accumulation in cold ischemic/reperfused rat liver. Hepatology 1997; 26: 113–120.
Jaeschke H, Farhood A, Smith CW . Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J 1990; 4: 3355–3359.
Peralta C, Jimenez-Castro MB, Gracia-Sancho J . Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. J Hepatol 2013; 59: 1094–1106.
Shin T, Kuboki S, Lentsch AB . Roles of nuclear factor-κB in postischemic liver. Hepatol Res 2008; 38: 429–440.
Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 1996; 274: 1379–1383.
Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS . NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998; 281: 1680–1683.
Luedde T, Assmus U, Wustefeld T, Meyer zu Vilsendorf A, Roskams T, Schmidt-Supprian M et al. Deletion of IKK2 in hepatocytes does not sensitize these cells to TNF-induced apoptosis but protects from ischemia/reperfusion injury. J Clin Invest 2005; 115: 849–859.
Rao J, Qian X, Wang P, Pu L, Zhai Y, Wang X et al. All-trans retinoic acid preconditioning protects against liver ischemia/reperfusion injury by inhibiting the nuclear factor κB signaling pathway. J Surg Res 2013; 180: e99–e106.
Suetsugu H, Iimuro Y, Uehara T, Nishio T, Harada N, Yoshida M et al. Nuclear factor κB inactivation in the rat liver ameliorates short term total warm ischaemia/reperfusion injury. Gut 2005; 54: 835–842.
Xu MQ, Shuai XR, Yan ML, Zhang MM, Yan LN . Nuclear factor-κB decoy oligodeoxynucleotides attenuates ischemia/reperfusion injury in rat liver graft. World J Gastroenterol 2005; 11: 6960–6967.
Lee YR, Hwang JK, Lee HS, Cheon YJ, Ryu JH, Lee SI et al. SPA0355, a thiourea analogue, inhibits inflammatory responses and joint destruction in fibroblast-like synoviocytes and mice with collagen-induced arthritis. Brit J Pharmacol 2011; 164: 794–806.
Bae UJ, Song MY, Jang HY, Jin Gim H, Ryu JH, Lee SM et al. The efficacy of SPA0355 in protecting β cells in isolated pancreatic islets and in a murine experimental model of type 1 diabetes. Exp Mol Med 2013; 45: e51.
Jaeschke H, Lemasters JJ . Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 2003; 125: 1246–1257.
Llacuna L, Mari M, Lluis JM, Garcia-Ruiz C, Fernandez-Checa JC, Morales A . Reactive oxygen species mediate liver injury through parenchymal nuclear factor-κB inactivation in prolonged ischemia/reperfusion. Am J Pathol 2009; 174: 1776–1785.
Matsui N, Kasajima K, Hada M, Nagata T, Senga N, Yasui Y et al. Inhibiton of NF-κB activation during ischemia reduces hepatic ischemia/reperfusion injury in rats. J Toxicol Sci 2005; 30: 103–110.
Kuboki S, Sakai N, Clarke C, Schuster R, Blanchard J, Edwards MJ et al. The peptidyl-prolyl isomerase, Pin1, facilitates NF-κB binding in hepatocytes and protects against hepatic ischemia/reperfusion injury. J Hepatol 2009; 51: 296–306.
Okaya T, Lentsch AB . Hepatic expression of S32A/S36A IκBα does not reduce postischemic liver injury. J Surg Res 2005; 124: 244–249.
Yu J, Lee HS, Lee SM, Yu HC, Moon WS, Chung MJ et al. Aggravation of post-ischemic liver injury by overexpression of A20, an NF-κB suppressor. J Hepatol 2011; 55: 328–336.
He SQ, Zhang YH, Venugopal SK, Dicus CW, Perez RV, Ramsamooj R et al. Delivery of antioxidative enzyme genes protects against ischemia/reperfusion-induced liver injury in mice. Liver Transplant 2006; 12: 1869–1879.
Suzuki M, Takeuchi H, Kakita T, Unno M, Katayose Y, Matsuno S . The involvement of the intracellular superoxide production system in hepatic ischemia-reperfusion injury. In vivo and in vitro experiments using transgenic mice manifesting excessive CuZn-SOD activity. Free Radic Biol Med 2000; 29: 756–763.
Schauer RJ, Gerbes AL, Vonier D, Meissner H, Michl P, Leiderer R et al. Glutathione protects the rat liver against reperfusion injury after prolonged warm ischemia. Ann Surg 2004; 239: 220–231.
Uchida Y, Freitas MC, Zhao D, Busuttil RW, Kupiec-Weglinski JW . The protective function of neutrophil elastase inhibitor in liver ischemia/reperfusion injury. Transplantation 2010; 89: 1050–1056.
Akahori T, Sho M, Hamada K, Suzaki Y, Kuzumoto Y, Nomi T et al. Importance of peroxisome proliferator-activated receptor-γ in hepatic ischemia/reperfusion injury in mice. J Hepatol 2007; 47: 784–792.
Chen YX, Sato M, Kawachi K, Abe Y . Neutrophil-mediated liver injury during hepatic ischemia-reperfusion in rats. Hepatob Pancreat Dis 2006; 5: 436–442.
Ke B, Shen XD, Gao F, Qiao B, Ji H, Busuttil RW et al. Small interfering RNA targeting heme oxygenase-1 (HO-1) reinforces liver apoptosis induced by ischemia-reperfusion injury in mice: HO-1 is necessary for cytoprotection. Hum Gene Ther 2009; 20: 1133–1142.
Acknowledgements
This work was supported by the Bio & Medical Technology Development Program (No. NRF-2012M3A9B2027975) and the Medical Research Center Program (No. 2008-0062279 and 2011-0030074) through the National Research Foundation (NRF) funded by the Korean government (MSIP).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Bae, UJ., Yang, J., Ka, SO. et al. SPA0355 attenuates ischemia/reperfusion-induced liver injury in mice. Exp Mol Med 46, e109 (2014). https://doi.org/10.1038/emm.2014.48
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2014.48
This article is cited by
-
oxLDL inhibits differentiation of mesenchymal stem cells into osteoblasts via the CD36 mediated suppression of Wnt signaling pathway
Molecular Biology Reports (2019)
-
Preparation of a steroid-oxazole-1,2′-[1,3]oxazete] derivative: biological and theoretical evaluation of its interaction with a kinase protein (CK2)
SN Applied Sciences (2019)
-
The protein kinase 2 inhibitor tetrabromobenzotriazole protects against renal ischemia reperfusion injury
Scientific Reports (2015)
-
Aggravation of post-ischemic liver injury by overexpression of insulin-like growth factor binding protein 3
Scientific Reports (2015)