Abstract
Epidemiological studies have suggested an association between pesticide exposure and Parkinson's disease. In this study, we examined the neurotoxicity of an organochlorine pesticide, heptachlor, in vitro and in vivo. In cultured SH-SY5Y cells, heptachlor induced mitochondria-mediated apoptosis. When injected into mice intraperitoneally on a subchronic schedule, heptachlor induced selective loss of dopaminergic neurons in the substantia nigra pars compacta. In addition, the heptachlor injection induced gliosis of microglia and astrocytes selectively in the ventral midbrain area. When the general locomotor activities were monitored by open field test, the heptachlor injection did not induce any gross motor dysfunction. However, the compound induced Parkinsonism-like movement deficits when assessed by a gait and a pole test. These results suggest that heptachlor can induce Parkinson's disease-related neurotoxicities in vivo.
Similar content being viewed by others
Introduction
It has long been recognized that both environmental and genetic factors contribute to the development of Parkinson’s disease (PD). Neurotoxicants, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxydopamine and pesticides, have been implicated in PD pathogenesis.1 Epidemiological studies have suggested that exposures to pesticides, such as rotenone, paraquat and maneb, can be a risk factor for PD.2, 3 In addition to case–control or cohort studies, neurotoxicological data from cell culture or animal models also indicate that pesticides can induce PD-associated behavioral and toxicological changes.4, 5
The insecticide rotenone is a specific inhibitor of mitochondrial electron transport chain (ETC) complex I and has been shown to induce PD-mimetic behavioral and brain histochemical changes in rodents as well as dopaminergic neuronal death in vitro.2, 5 Paraquat, an herbicide with relatively weak mitochondrial ETC complex I inhibitory activity, has also been shown to exert cytotoxicity on dopaminergic neurons.5 Although paraquat is structurally very similar to MPTP, its toxicity mechanism is reported to be different from MPTP or even from another complex I inhibitor, rotenone.6 It has been suggested that paraquat induces oxidative stress as a redox cycler in the cytoplasm and damages the mitochondria indirectly.7 The PD-mimetic behavioral change in rodents induced by paraquat was more prominent when treated in combination with maneb.8 Maneb is a fungicide known to inhibit mitochondrial complex III.9 Although these toxin models of PD do not perfectly recapitulate human PD pathology, they have contributed to our current understanding of the mechanism of PD pathogenesis via mitochondrial damage and oxidative stress.
Another class of pesticide suggested as an environmental toxicant related with PD is organochlorine compound.10 Among organochlorine pesticides, dieldrin and heptachlor have been examined for its PD-related neurotoxicities.10 Dieldrin has been detected in the postmortem brain tissues of PD patients.11, 12 In cell culture studies, it has been shown that dieldrin induces oxidative stress and mitochondria-mediated apoptosis.13 Another organochlorine pesticide, heptachlor, has been implicated as a possible PD-related neurotoxicant. Mouse model studies have suggested that exposures to heptachlor induced changes in the expression of dopamine transporters, which may alter the susceptibility of dopaminergic neurons to other PD-promoting neurotoxicants.14, 15, 16 Recently, we have reported that heptachlor can inhibit the mitochondrial ETC complex III and thus promote the generation of reactive oxygen species in cultured dopaminergic cells.17 We found that the oxidative stress induced by mitochondrial complex III blockade by heptachlor may activate Bax- and mitochondria-mediated apoptosis.17 Our in vitro study suggesting a neurotoxicity mechanism for heptachlor raises a possibility that heptachlor can act as a direct neurotoxicant on the dopaminergic neurons in the substantia nigra (SN). However, it has not been addressed whether heptachlor can induce direct neurotoxicity on the nigral dopaminergic neurons in vivo.
In rodent models of PD, neurotoxic compounds, such as MPTP and 6-hydroxydopamine, induced not only degeneration of the nigral dopaminergic neurons but also motor deficits mimicking clinical symptoms of PD. Impairments in motor performance as displayed in PD patients can be monitored in rodent models by various behavioral tests such as cylinder, beam traversal, pole, grid or adhesive removal test.18 Among these behavioral tests, the pole test has been known to be a very sensitive method that can detect nigrostriatal dysfunction.18, 19 In the pole test, a mouse is placed head up near the top of a thin pole, and the time to turn and climb down is measured.19 This test monitors the forepaw dexterity of mice and is reported to be highly correlated with striatal dopamine content.20 Thus, the pole test has been used to detect bradykinesia.19, 20
In this study, we examined whether heptachlor can induce degeneration of the nigral dopaminergic neurons. We observed selective loss of dopaminergic neurons as well as gliosis in the SN when heptachlor was intraperitoneally injected into mice on a subchronic schedule. Furthermore, the heptachlor injection induced subtle but clear deficits in motor function when examined by pole test and gait test. Our observations suggest that heptachlor can act as a direct neurotoxicant promoting PD pathogenesis.
Materials and methods
Antibodies and reagents
Anti-glial fibrillary acidic protein, anti-CD11b, anti-α actin and anti-tyrosine hydroxylase (TH) antibodies were from Millipore (Billerica, MA, USA). Anti-glutamic acid decarboxylase (GAD) 65, cytochrome c oxidase IV subunit 1 (COX IV) and anti-cytochrome c antibodies were from Abcam (Cambridge, UK). JC-1 dye and MitoTracker Red 580 were from Invitrogen (Carlsbad, CA, USA). Heptachlor was purchased from Supelco (St Louis, MO, USA). Fluorogenic substrates of caspases were all purchased from Bachem (Torrance, CA, USA). Cell culture media and supplements were purchased from JBI (Daegu, Korea). All other reagents were purchased from Sigma (St Louis, MO, USA) unless stated otherwise.
Measurement of mitochondrial membrane potential
Mitochondrial membrane potential was determined by flow cytometry using the lipophilic cationic fluorochrome JC-1 (Invitrogen). Cells were incubated with JC-1 (5 μg ml–1) at 37 °C for 30 min. After washing three times with cold phosphate-buffered saline (PBS), cells were fixed in 4% paraformaldehyde. Flow cytometric analysis was performed on fluorescence-activated cell sorting caliber using Cell Quest software (Becton Dickinson Biosciences, Franklin Lakes, NJ, USA). JC-1 monomer (green fluorescence) and aggregates (red fluorescence) were detected separately in FL1 (λem=527) and FL2 (λem=590) channel, respectively.
Immunocytochemistry
For immunocytochemistry, cells were fixed with 4% paraformaldehyde in PBS (137 mM of NaCl, 2.7 mM of KCl, 100 mM of Na2HPO4, 2 mM of KH2PO4) for 10 min and permeabilized in 0.5% PBST (0.5% Triton X-100 in PBS) for 10 min. After permeabilization, the cells were incubated in 0.1% PBST containing 10% normal goat serum (Vector Laboratories, Burlingame, CA, USA) for 1 h. After blocking, the cells were incubated with primary antibodies diluted in 0.1% PBST with 2% normal goat serum at 4 °C overnight. Following washing with 0.1% PBST four times for 5 min each, the samples were incubated with matching secondary antibodies conjugated with biotin for 1 h. After being washed four times, biotin-decorated samples were incubated with fluorescein isothiocyanate- or Texas red-conjugated streptavidin (Invitrogen) for 30 min at room temperature. For mitoTracker staining, cells were incubated with mitoTracker Red 580 (100 nM) for 30 min. After washing, the cells were fixed with 4% paraformaldehyde. For annexin-V staining, cells were incubated with annexin-V-biotin solution (Roche, Basel, Switzerland) for 15 min, washed and then fixed. Then, the cells were incubated with fluorescein isothiocyanate-conjugated streptavidin for 30 min, followed by washing. The stained cells were mounted with medium containing 4′,6-diamidino-2-phenylindole (Slowfade Gold antifade reagent with 4′,6-diamidino-2-phenylindole, Invitrogen). The samples were examined under a fluorescence microscope (Axioplan 2, Zeiss, Oberkochen, Germany).
Subcellular fractionation
The cells were homogenized in a pre-chilled lysis buffer containing 10 mM HEPES, 50 mM NaCl, 0.1 mM EDTA, 0.5 M Sucrose, 0.5% Triton X-100 (pH 7.9) supplemented with 1 mM dithiothreitol and a protease inhibitor cocktail (Roche). Following incubation on ice for 20 min, the lysates were centrifuged at 10 000 × g. The supernatant was designated as cytosolic fraction. The pellet was resuspended with the lysis buffer and centrifuged at 10 000 × g for 20 min. With the second supernatant discarded, the pellet was resuspended and designated as heavy membrane fraction. The fractionated lysates were processed for the measurement of protein concentration by bicinchoninic acid assay according to the manufacturer’s protocol (Pierce, Rockford, IL USA).
Immunoblot assay
To monitor the GAD65 level, SN tissues were dissected under a dissection microscope and homogenized in a pre-chilled lysis buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1% TritonX-100 with a protease inhibitor cocktail. Cultured cells were lysed using immunoprecipitation lysis buffer containing 25 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 5% glycerol with a protease inhibitor cocktail. Following protein quantification by bicinchoninic acid assay, the tissue lysates or cell culture lysates were processed according to the standard protocol for the immunoblot assay.
Immunohistochemistry
One week after the last injection, the mice were anesthetized with isoflurane and transcardially perfused with pre-chilled PBS and then 4% paraformaldehyde in PBS (pH 7.4). The brains were removed, dissected using mouse coronal brain matrices (Applied Scientific Instrumentation, Eugene, OR, USA), and embedded in optimum cutting temperature compound (VWR, Randor, PA, USA) and then stored at −80 °C. Coronal sections (10 μm thick) corresponding to the SN level (bregma −2.70 to −3.70) and striatum level (bregma 1.8 to −2.1) were prepared using a cryotome (CM3000, Leica, Wetzlar, Germany) and fixed with 4% paraformaldehyde in PBS for 10 min. After rinsing and quenching endogenous peroxidase with 0.1% H2O2 in deionized water for 10 min, the samples were incubated in PBST with 10% normal goat serum for 1 h. Then, the sections were incubated with primary antibodies in 0.1% PBST containing 2% normal goat serum overnight at 4 °C. Rabbit polyclonal anti-TH for dopaminergic neurons (1:500), mouse monoclonal anti-GAD65 for GABAergic neurons (1:1000), mouse monoclonal anti-glial fibrillary acidic protein for astrocytes (1:200) and rat monoclonal anti-CD11b for microglia (1:200) were used. The decorated antibodies were visualized by the ABC method according to the manufacturer’s protocol (ABC Elite kit, Vector Laboratories). For the quantitative measurement of staining intensities, photomicrographs were analyzed using ImageJ software (NIH, Bethesda, MD, USA).
Caspase activity assay
To examine enzyme activities of caspases, the cleavage of YVAD-amc, DEVD-amc, IETD-amc or LEHD-amc was monitored in the lysates of the dissected brain tissues at indicated time points. The brain (cortex, SN and cerebellum) was snap-frozen in liquid nitrogen and pulverized in a liquid nitrogen-chilled mortar. The lysis buffer was added to the pulverized tissue powder and the lysates were incubated for 30 min at 4 °C with agitation. The lysates were centrifuged at 10 000 × g for 20 min. Then, the supernatant was measured for protein concentration using a bicinchoninic acid assay (Pierce). Fifty micrograms of the tissue lysates supernatant were incubated with each caspase substrate (40 μM) in 100 mM HEPES buffer containing 1 mM dithiothreitol in a total volume of 50 μl for 10 min at 37 °C. After preincubation, fluorescence as a result of caspase activation was measured for 20 min at 30-s intervals using a spectrofluorometer (SpectraMax Gemini EM, Molecular Devices, Sunnyvale, CA, USA). Fluorescence was read at λex=355 nm and λem=460 nm. A slope from the fluorescence increase was defined as relative caspase activity.
Primary cultures
Cortical neurons were cultured from E14 mouse embryo (C57BL/6). The cortices were dissected, minced and incubated in Hank’s balanced salt solution with 0.1% trypsin-EDTA for 10 min at 37 °C. Following trituration in Dulbecco’s modified Eagle’s medium supplemented with 15% fetal bovine serum, the cells were plated at a density of 5 × 105 cells ml–1 in Neurobasal media supplemented with B27 and Glutamax-1. For near-pure neuronal cultures, cytosine arabinoside (AraC, 5 μM) was added 3 days after plating. The neurons were used for experiments 6 days after plating. For the neuronal/glial co-cultures, the cells were maintained without the addition of AraC. Mixed glial cells were cultured from P1 mouse pups (C57BL/6). Three hours after the first plating of the dissociated cells, the medium was changed to remove the unattached cells. The attached glial cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum for 6 days until use. Cell death of the cultured cells was assessed by MTS assay using CellTiter 96 AQueous One Solution Assay kit (Promega, Madison, WI, USA).
Reverse transcription polymerase chain reaction
Total RNA was isolated from the cultured cells using RNeasy minikit (Qiagen, Valencia, CA, USA). Complementary DNA was synthesized using the Moloney Murine Leukemia Virus Reverse Transcriptase (Promega) according to the protocol of the manufacturer. The PCR primers are as follows. Microtubule-associated protein 2 (404 bp) forward: 5′-GAAGGAAAGGCACCACACTG-3′, reverse: 5′-GCTGGCGATGGTGGTGGG-3′; glial fibrillary acidic protein (346 bp) forward: 5′-TTGCAGACCTCACAGACGCTGCGT-3′, reverse: 5′-CGGTTTTCTTCGCCCTCCAGCAAT-3′; cyclooxygenase-2 (Cox-2, 150 bp) forward: 5′-GCTGCCCGACACCTTCAACATT-3′, reverse: 5′-CACATTTCTTCCCCCAGCAACC-3′; beta-actin (322 bp) forward: 5′-GTATGGAATCCTGTGGCATC-3′, reverse: 5′-AAGCACTTGCGGTGCACGAT-3′. PCR was performed using standard protocol for 27 cycles at an annealing temperature of 56 °C.
Animal care and heptachlor injection
All experiments on mice were performed according the guidelines of the IACUC at Sejong University, Seoul, Republic of Korea. Mice were group-housed under a 12:12 light–dark lighting schedule with free access to food and water. Temperature in the room was 22 °C.
Twelve- to 16-week-old male C57BL/6 mice were injected intraperitoneally with 0.5% dimethylsulphoxide (100 μl each, n=8) or heptachlor (7 mg kg−1 in a volume of 100 μl 0.5% dimethylsulphoxide each, n=8) twice a week for 8 weeks. The LD50oral and LD50intraperitoneal of heptachlor for mice are known to be 70 mg kg−1 and 130 mg kg−1, respectively.21 Body weight was measured before every injection. Behavioral tests were performed 1 week after the last injection.
Open field test
To monitor general activity levels, gross locomotor activity and explorative behaviors, a mouse was placed in the center of the open field arena and allowed to move freely for 20 min while being tracked by an automated tracking system (Ethovision, Noldus, Wageningen, The Netherlands). Time of stay in the center or the corner area, rearing activity, total distance moved and velocities were recorded for 20 min. Before each recording, a mouse was allowed to explore the arena to acclimatize to the space. The recordings were analyzed by Ethovision.
Pole test
A pole test19 was performed to evaluate bradykinesia of the heptachlor-injected mice. A mouse was positioned at the top of a rough-surfaced pole (10-mm diameter and 60-cm height), heads up, and then the time to turn and reach the floor was measured. In a habituation period 1 day before testing, each mouse is allowed to attempt to descend the pole. On the test day, mice were allowed to practice five times and then tested. The time to turn was first measured from the beginning of movement until the mouse turns completely heads down, and time to reach the floor was measured until the mouse arrives at the floor. Each testing session consisted of three trials; each trial lasted for a maximum of 180 s. Each mouse was tested twice before the 8-week drug injection period and following 1 week after the final injection.
Gait distance measurement
Mice were first trained to walk through a dark tunnel, which was then lined with a clean white paper. The two front feet were then dipped into non-toxic ink, and the mouse was allowed to walk through the tunnel again. After the feet washing, the mice were returned to the home cage. Once the footprint was dried, the paper was scanned and the distances between the footprints were measured.
Statistics
For the statistical analysis, all the experiments were repeated at least three times. The results were expressed as the means±s.d. of at least three independent experiments, unless stated otherwise. Data from open field test were evaluated by analysis of variance. All other data were evaluated by Student’s t-test.
Results
Heptachlor induced mitochondria-mediated cell death in SH-SY5Y cells
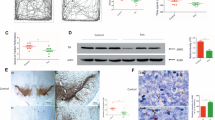
We have recently shown that heptachlor inhibits the mitochondrial ETC complex III activity and promotes the generation of reactive oxygen species to induce Bax-mediated apoptosis in the human dopaminergic cell line SH-SY5Y.17 To further confirm whether heptachlor induces mitochondria-mediated apoptosis, we monitored the mitochondrial membrane potential using JC-1 dye because the membrane potential is a sensitive indicator of the mitochondrial status.22 SH-SY5Y cells were treated with heptachlor for the indicated times and then incubated with JC-1 for 30 min, followed by fluorescence-activated cell sorting analysis. As shown in Figure 1a, heptachlor started to induce a decrease in mitochondrial membrane potential as indicated by an increase in green fluorescence at 1 h after the treatment. The decrease in the membrane potential peaked 4 h after the treatment. When the ratio of JC-1 red aggregates to green monomer was determined from the fluorescence-activated cell sorting analysis, the cells that were treated with heptachlor for 4 h exhibited approximately 50% reduction in the red:green ratio compared with the vehicle-treated cells (Figure 1b). This clearly indicates that heptachlor induced mitochondrial potential decrease and is consistent with our previous observation of the decrease in cellular ATP level by heptachlor.17
Heptachlor induced mitochondria-mediated cell death in SH-SY5Y cells. (a) To monitor mitochondrial potential, cells were treated with heptachlor (80 μM), followed by incubation with JC-1 dye (5 μM). A shift in fluorescence was detected by fluorescence-activated cell sorting (FACS) analysis and the changes in green fluorescence (FL1-H) are shown. (b) The ratio of JC-1 red aggregates to green monomer was calculated from the FACS analysis 4 h after the heptachlor treatment (mean±s.d., n=4, **P<0.01 vs vehicle). (c) To examine the changes in the cytochrome c localization, the heptachlor-treated cells were stained with mitotracker (red) and then anti-cytochrome c antibody (green, anti-cyt. c). (d) To confirm the cytochrome c release from the mitochondria, the heptachlor-treated cells (80 μM, 8 h) were fractionated into heavy membrane (HH) and cytosolic (Cytosol) fractions and subjected to immunoblots using anti-cytochrome c (Cyt. c) antibody. Blots for cytochrome c oxidase IV subunit 1 (COX IV) served as a fractionation control. (e) SH-SY5Y cells were treated with heptachlor (HPC, 80 μM) and then processed for Annexin V staining at 18 h after the incubation. Representative images showing nuclear morphology of the heptachlor-treated SH-SY5Y cells as stained by 4′,6-diamidino-2-phenylindole (DAPI; top panels). Note the condensed and fragmented nuclei (arrowheads). The heptachlor-incubated cells were stained with fluorescein isothiocyanate (FITC)-annexin V (bottom panels). (f) The rate of apoptotic cells was calculated by quantifying fragmented or condensed nuclei following a 12-h heptachlor treatment (40 and 80 μM). Means±s.d. are shown (n=5, **P<0.01 and ***P<0.001 vs vehicle).
We then examined whether heptachlor can induce cytochrome c release. It is widely accepted that the decrease in mitochondrial membrane potential induces membrane permeability transition pore opening and the release of pro-apoptotic molecules such as cytochrome c into the cytosol.22 As shown in Figure 1c, immunoreactivity for cytochrome c in the heptachlor-treated cells was diffuse throughout the cytoplasm whereas the immunoreactivity in the vehicle-treated cells was confined in the typical filamentous structure of mitochondria. Furthermore, when the heptachlor-treated cells were fractionated into the heavy membrane and cytosolic fractions, it was evident that a fair amount of cytochrome c was detected in the cytosolic fractions (Figure 1d). This result indicates that heptachlor induced cytochrome c release from the mitochondria following mitochondrial permeability transition to induce mitochondria-mediated apoptosis. Previously, we observed that heptachlor induced caspase-9 and -3 activation and the cell death induced by this compound was efficiently suppressed by pan-caspase inhibitor, zVAD-fmk.17 These results clearly show that heptachlor-induced cell death is a caspase-dependent apoptosis. Indeed the shrunken or fragmented nuclei as revealed by 4′,6-diamidino-2-phenylindole staining following heptachlor treatment also suggested the apoptotic form of cell death (Figure 1e). Furthermore, the heptachlor-treated cells were stained with annexin V, which detects the exposed phosphatidyl serine at the later stage of apoptosis (Figure 1e, bottom panels). When the apoptotic nuclei was counted (Figure 1f), approximately 35% and 55% of the cells showed apoptotic nuclei following 40 μM and 80 μM heptachlor treatment, respectively. This is consistent with our previous results showing that a similar portion of cell death was inhibited by zVAD-fmk at each heptachlor concentration.17 Taken together, these results suggest that heptachlor can induce mitochondria-mediated apoptosis in SH-SY5Y cells.
Heptachlor induced selective degeneration of SN dopaminergic neurons
To examine whether heptachlor can induce degeneration of dopaminergic neurons in the SN pars compacta (SNpc) in vivo, C57BL/6 mice were injected with heptachlor intraperitoneally at a dose of 7 mg kg−1 body weight twice a week for 8 weeks, and the histological changes in the injected mouse brain were examined 1 week after the last injection. When the numbers of TH-positive neurons were counted in the SNpc area, it was evident that heptachlor induced a significant decrease in the TH-positive dopaminergic neuronal population (Figure 2a). The number of TH-positive cells in the SNpc of the heptachlor-injected mice was approximately 57% of the vehicle-injected mice. The vehicle, 0.5% dimethylsulphoxide, did not induce any loss of the TH-positive neurons in the examined area (Figure 2a). To examine whether the toxicity is selective for the TH-positive dopaminergic neurons, the samples were immunostained with antibodies against GAD65, a marker for GABAergic neurons. As shown in Figure 2b, the intensity of anti-GAD65 immunostaining at SN level did not differ significantly between the two groups. In addition, when the protein levels of GAD65 were compared by immunoblot assay using the SN tissue dissected from the vehicle- and heptachlor-injected mice, no significant difference was observed between the two groups (Figure 2c). These results suggest that the heptachlor-induced toxicities may be selective for the dopaminergic neurons that are reportedly more vulnerable to oxidative stress.23
Heptachlor induced selective degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc). To examine the in vivo effect of heptachlor in the brain, C57BL/6 mice were intraperitoneally injected with 7 mg kg–1 body weight of heptachlor (HPC) or 0.5% dimethylsulphoxide (DMSO; vehicle) twice a week for 8 weeks, and the signs of neurotoxicities were examined 1 week after the last injection. (a) To examine whether heptachlor can induce degeneration of dopaminergic neurons in the SNpc in vivo, the brain tissue sections at the substantia nigra (SN) level were prepared for the immunohistochemical staining using anti-tyrosine hydroxylase (TH) antibodies from uninjected naive, vehicle-injected and heptachlor-injected mice. Representative photomicrographs are shown (20 × objective). TH-positive neurons are shown in the SNpc as indicated by the dotted lines. Quantification of the TH-immunohistochemistry results is shown in the right panel. The numbers of TH-positive neurons were counted in the SNpc area (mean±s.d., n=5, six tissue sections from each mouse, ***P<0.001 vs vehicle or uninjected). (b) The brain sections at SN level were also immunostained with anti-GAD65 antibody. Quantification of the staining intensities is shown (n=5). (c) Immunoblotting using GAD65 antibody was performed on the brain tissue lysates prepared from the vehicle- or heptachlor-injected mice. (d) TH immunohistochemistry was performed on the striatum and the quantification of the immunoreactivity intensity readings is shown (v4, six tissue sections from each mouse, **P<0.01 vs vehicle). (e) To examine whether heptachlor induced selective toxicities in the SN, the brains from the injected mice were dissected into cerebellum, SN, and cortical area for the measurement of fluorogenic substrate-cleaving activities for caspase-1 (YVAD), -3 (DEVD) and -9 (LEHD). Data are expressed as the mean±s.d. (n=5). NS, not significant.
It is known that TH level as well as dopamine level is also reduced in the PD animal models.8, 24 To examine whether the striatum was also affected by the heptachlor injection, the brain sections at the striatum level were examined by immunohistochemistry using anti-TH antibody. As shown in Figure 2d, the striatum of the heptachlor-injected mice exhibited significantly reduced level of TH immunoreactivity compared with the vehicle-injected groups. This suggests that the striatum as well as SN was affected by the heptachlor-induced damage.
To further examine whether heptachlor induced selective toxicity in the SN leading to apoptotic cell death, activities of caspases were monitored in the different brain areas of the heptachlor-injected mice. The brains from the injected mice were dissected into cerebellum, cortex and SN for the measurement of fluorogenic substrate-cleaving activities for caspase-1, -3 and -9. Figure 2e shows that all three of the substrate-cleaving activities were significantly elevated in the SN tissue lysates, whereas those of cerebellum and cortex remained basal or slightly increased. This result further suggests that heptachlor may have selective toxicity against SN and induced caspase-mediated apoptosis in vivo.
Heptachlor induced gliosis in the SN area
It is well documented that gliosis accompanies neuronal degeneration during the pathogenesis of neurotoxin-induced Parkinsonism model in mice.24 To examine whether heptachlor induced gliosis in the injected mice, the brains were analyzed by immunohistochemistry using anti-glial fibrillary acidic protein and anti-CD11b antibodies to detect astrogliosis and microgliosis, respectively. As shown in Figure 3a and quantified on the right panels, both reactive astrocytes and microglia were concentrated in the SN of the heptachlor-injected mice but not in the vehicle-injected mice. However, cortical and hippocampal area did not show any difference in the levels of gliosis between the heptachlor- and vehicle-injected groups. The cortical area exhibited very low levels of both the glia in the heptachlor- and vehicle-injected groups. Hippocampal area showed higher levels of astrocytes and microglia compared with the cortical area, but the levels of glial population did not differ between the two groups. These results imply that heptachlor may have induced selective degeneration in the SN to induce gliosis in the affected area.
Heptachlor induced gliosis selectively in the ventral midbrain area. (a) To monitor possible gliosis, brain sections at indicated brain levels (substantia nigra (SN), cortex and hippocampus) were immunostained with anti-glial fibrillary acidic protein (GFAP) or anti-CD11b antibodies. Representative photomicrographs are shown (20 × objective). Quantification of the immunoreactivity readings is shown on the right panels (mean±s.d., n=5, four tissue sections from each mouse, **P<0.01 and ***P<0.001 vs vehicle). (b–c) Cell death rate of cultured near-pure cortical neurons (b) or mixed glia (c) was determined by MTS assay following vehicle or heptachlor treatment at indicated concentrations for 12 h (n=3, ***P<0.001 vs vehicle). Representative cell images are shown in the right panels. (d) Induction of cyclooxygenase-2 (Cox-2) mRNA was examined by reverse transcriptase-PCR (RT-PCR) in the near-pure neurons (neuron), mixed glia (glia) and neuron/glia co-cultures (co-culture) following vehicle (V) treatment for 8 h or 25 μM heptachlor for 8 and 12 h. RT-PCR for microtubule-associated protein 2 (MAP2) and glial fibrillary acidic protein (GFAP) served as a culture purity control. RT-PCR for actin served as an internal control. (e) RT–PCR analysis of Cox-2 mRNA in the glial cells following 50 and 100 μM heptachlor treatment for 8 and 12 h.
We then wanted to determine whether heptachlor induced inflammatory response by directly stimulating glial cells or indirectly inducing neuronal damage. First, we assessed the responsive concentration range of primary neurons and glia to heptachlor. For this, the cultured cells were treated with varying concentrations of heptachlor, and the rate of cell death was monitored by MTS assay. As shown in Figure 3b, near-pure cortical neurons were more sensitive to heptachlor than SH-SY5Y cells, and approximately 90% of the cells died after 25 μM of heptachlor treatment. However, mixed glial cells were much more resistant and the viability was not affected even with 100 μM of heptachlor (Figure 3c). To gain a clue how heptachlor induced inflammation in the SN in vivo, the induction of a proinflammatory gene, Cox-2, was examined by reverse transcriptase-PCR in the near-pure neurons, glia and neuron/glia co-cultures following treatment with varying concentrations of heptachlor (Figures 3d and e). Cox-2 is an inducible proinflammatory molecule reported to be involved in the pathogenesis of PD.25 As shown in Figure 3d, at the heptachlor concentration (25 μM) where only neurons were vulnerable, the induction of Cox-2 was evident only in the neuron/glia co-cultures but not in neuron-only or glia-only cultures. However, higher concentrations of heptachlor did induce Cox-2 in the glia-only cultures (Figure 3e). These results suggest that heptachlor can affect glial cells directly to induce inflammation at a higher concentration, but at lower concentrations it may induce inflammation indirectly by damaging neurons. Taken together, our in vivo and in vitro results suggest a possibility that heptachlor induced neuronal damage and this in turn induced gliosis and inflammation.
Heptachlor induced Parkinsonism-related behavioral changes
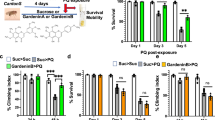
As we observed the loss of dopaminergic neurons and gliosis in the SN in the heptachlor-injected mice, we wanted to determine whether heptachlor can induce motor dysfunction mimicking Parkinsonism. When the changes in the body weights were monitored, the two groups did not show any significant difference during the injection period (Figure 4a). This suggests that the injected heptachlor did not induce general toxicity that may be reflected as weight loss. One week after the last injection, the general locomotor activities of the uninjected, vehicle- or heptachlor-injected mice were monitored by open field test. There were no differences in the total distance moved, mean velocity and rearing frequency among the groups tested (Figures 4b–d). In addition, the level of anxiety as monitored by center duration in the open field was also normal in the heptachlor-injected mice (Figure 4e).
Heptachlor did not affect general locomotor activity. To examine whether the injection of heptachlor can induce Parkinsonian behavioral changes, mice were injected with 7 mg kg–1 body weight of heptachlor or 0.5% dimethylsulphoxide (DMSO; vehicle) twice a week for 8 weeks. Body weight was measured before every injection for the 8 weeks (a). One week after the last injection, general locomotor activities (b–d) and anxiety level (e) were assessed (n=5 each). Total distance moved (b), mean velocity (c) and rearing frequency (d) were monitored by open field test. (e) The level of anxiety as monitored by center duration in the open field was also monitored. No statistically significant differences were detected between the three groups upon analysis of variance analysis (b–e).
Confirming that heptachlor did not induce general weakness or change the level of anxiety that may affect motor performance, we then performed a pole test where a mouse is placed on top of a rod with the head pointing upward, and then the time to turn downward and time to reach the floor are measured for each mouse. It has been reported that the motor performance on the pole test is dependent on the striatal dopamine content.19 Thus, pole test has been used as a sensitive method to detect nigrostriatal dysfunction.18 When the heptachlor-injected mice were tested on the pole, they spent significantly more time to turn downward on the pole than the vehicle-injected mice (Figure 5a). It took about 7 s for the vehicle-injected and uninjected mice to turn downward on the pole top, but it took approximately 12 s for the heptachlor-injected ones.
Heptachlor induced Parkinsonism-like movement deficits. (a) To assess the forepaw dexterity, the injected mice were subjected to a pole test. The time to turn downward on the test rod was measured before and 1 week after the 8-week injections. (b) To determine whether there is a gait disturbance, the stride length was measured before and after the injection for each mouse. *P<0.05 vs before the injections (n=5 each, Student’s t-test).
To investigate further whether heptachlor induced Parkinsonism-related behavioral changes in the injected mice, we measured the stride length following the 2-month injection period. As shown in Figure 5b, the mice injected with heptachlor showed shortened stride length when compared with the vehicle-injected mice. The average stride length of the heptachlor-injected mice was shortened by approximately 15% after the injection (n=6, P<0.05). Taken together, these results suggest that heptachlor induced subtle motor dysfunction, which is known to be associated with Parkinsonism but not general locomotor activity.
Discussion
Our study demonstrated that heptachlor can induce dopaminergic neuronal loss and behavioral deficits in mice when injected subchronically. In the case of dieldrin, intraperitoneal injection did not induce dopaminergic neuronal loss in the SN or behavioral changes, although it induced other damages related with PD.10 Other pesticides, such as rotenone or paraquat, were shown to induce dopaminergic neuronal loss and behavioral changes.26 Rotenone-infused rats exhibited impairments in locomotor activity assessed by an open field test.27 In our study, intraperitoneally injected heptachlor did not induce impairments in general locomotor activity in mice when the mean velocity, total distance moved and rearing activities were measured in the open field (Figures 4b–d). Considering the known LD50intraperitoneal of heptachlor is 130 mg kg–1 in rodents,21 the dose of 7 mg kg–1 that we used in this study does not seem to be high enough to induce general toxicities or weakness. The normal body weight increase in the injected mice (Figure 4a) further supports this conclusion. Previous mouse model studies reported that MPTP administration induced little or no impairment in movement when assessed by open field test, although MPTP did deplete dopamine in the nigrostriatal system.18 Thus, open field activities may not reflect the movement deficits induced by dopamine depletion. However, the injected mice took significantly more time to turn on a pole test. The pole test has been used as a sensitive method to evaluate grip strength and forepaw dexterity.19 It has been suggested that movement disorder in mice caused by striatal dopamine depletion can be detected by this test.20
Moreover, in accordance with the case of MPTP model of PD,28 heptachlor-injected mice exhibited shortened gait distance. Our results from the behavioral tests suggest that the systemic injection of heptachlor induced mild but clear Parkinsonism-like movement deficits. It was suggested that Parkinsonian symptoms become evident when approximately 70% of the dopaminergic neurons in the SNpc are lost.29 However, in the heptachlor-injected mice, we observed approximately 43% dopaminergic neuronal loss in the SNpc (Figure 2a). Thus, it is possible that the degree of dopaminergic neuronal loss was reflected in the severity of motor deficits. As heptachlor induced very specific and subtle motor deficits mimicking behavioral symptoms of PD, it is possible to use heptachlor injection as a model system to study the pathogenesis of PD.
Recently, we have shown that heptachlor inhibited mitochondrial ETC complex III activity and promoted the generation of reactive oxygen species, which might activate Bax to induce caspase-dependent apoptosis in SH-SY5Y cells.17 In this study, we have further confirmed that heptachlor induced mitochondria-mediated apoptosis by showing mitochondrial membrane potential decrease, cytochrome c release and an apoptotic form of cell death in SH-SY5Y cells. In accordance with this in vitro data, heptachlor induced caspase-9 and -3 activation much more prominently in the SN than in other brain areas, implying that mitochondria-mediated caspase activation occurred in the SN. The caspase-1 activity, as monitored by YVAD cleavage, may be due to the inflammatory response in vivo as evidenced by gliosis in the ventral midbrain area. In addition, a significant portion of TH-positive dopaminergic neurons was lost while GABAergic neurons were spared following the heptachlor injection. It has been suggested that dopaminergic neurons are more susceptible to mitochondrial damage and oxidative stress.23, 30 Therefore, it is possible that heptachlor induced selective toxicity in the nigral dopaminergic neurons due to the intrinsic property of the dopaminergic neurons. It remains to be studied whether there is a selective affinity or reuptake mechanism of heptachlor in the dopaminergic neurons.
In summary, we showed that heptachlor induced mitochondria-mediated cell death in SH-SY5Y cells and degeneration of SNpc dopaminergic neurons and gliosis in mice. In addition, the injected heptachlor induced motor deficits when assessed by gait or pole test. Although further studies are required to conclude that exposures to organochlorine pesticides are a causative factor for sporadic PD, our study suggests that heptachlor can contribute to the pathogenesis of PD.
References
Abdulwahid AI, Ahmad KH . Environmental toxins and Parkinson's disease: putative roles of impaired electron transport chain and oxidative stress. Toxicol Ind Health 2010; 26: 121–128.
Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS . Pesticides and Parkinson's disease—is there a link? Environ Health Perspect 2006; 114: 156–164.
Freire C, Koifman S . Pesticide exposure and Parkinson's disease: epidemiological evidence of association. Neurotoxicology 2012; 33: 947–971.
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT . Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 2000; 3: 1301–1306.
Cicchetti F, Drouin-Ouellet J, Gross RE . Environmental toxins and Parkinson’s disease: what have we learned from pesticide-induced animal models? Trends Pharmacol Sci 2009; 30: 475–483.
Berry C, La Vecchia C, Nicotera P . Paraquat and Parkinson's disease. Cell Death Differ 2010; 17: 1115–1125.
Bonneh-Barkay D, Langston WJ, Di Monte DA . Toxicity of redox cycling pesticides in primary mesencephalic cultures. Antioxid Redox Signal 2005; 7: 649–653.
Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA . The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson's disease. J Neurosci 2000; 20: 9207–9214.
Zhang J, Fitsanakis VA, Gu G, Jing D, Ao M, Amarnath V et al. Manganese ethylene-bis-dithiocarbamate and selective dopaminergic neurodegeneration in rat: a link through mitochondrial dysfunction. J Neurochem 2003; 84: 336–346.
Moretto A, Colosio C . The role of pesticide exposure in the genesis of Parkinson's disease: epidemiological studies and experimental data. Toxicology 2013; 307: 24–34.
Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR . Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol 1994; 36: 100–103.
Corrigan FM, French M, Murray L . Organochlorine compounds in human brain. Hum Exp Toxicol 1996; 15: 262–264.
Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V . Dieldrin-induced neurotoxicity: relevance to Parkinson's disease pathogenesis. Neurotoxicology 2005; 26: 701–719.
Kirby ML, Barlow RL, Bloomquist JR . Neurotoxicity of the organochlorine insecticide heptachlor to murine striatal dopaminergic pathways. Toxicol Sci 2001; 61: 100–106.
Caudle WM, Richardson JR, Wang M, Miller GW . Perinatal heptachlor exposure increases expression of presynaptic dopaminergic markers in mouse striatum. Neurotoxicology 2005; 26: 721–728.
Richardson JR, Caudle WM, Wang MZ, Dean ED, Pennell KD, Miller GW . Developmental heptachlor exposure increases susceptibility of dopamine neurons to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)in a gender-specific manner. Neurotoxicology 2008; 29: 855–863.
Hong S, Kim JY, Hwang J, Shin KS, Kang SJ . Heptachlor induced mitochondria-mediated cell death via impairing electron transport chain complex III. Biochem Biophys Res Commun 2013; 437: 632–636.
Meredith GE, Kang UJ . Behavioral models of Parkinson's disease in rodents: a new look at an old problem. Mov Disord 2006; 21: 1595–1606.
Ogawa N, Hirose Y, Ohara S, Ono T, Watanabe Y . A simple quantitative bradykinesia test in MPTP-treated mice. Res Commun Chem Pathol Pharmacol 1985; 50: 435–441.
Matsuura K, Kabuto H, Makino H, Ogawa N . Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods 1997; 73: 45–48.
Melnikov NN . Chemistry of pesticides. Residue Rev 1971; 36: 1–480.
Renault TT, Manon S . Bax: addressed to kill. Biochimie 2011; 93: 1379–1391.
Bueler H . Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson's disease. Exp Neurol 2009; 218: 235–246.
Meredith GE, Sonsalla PK, Chesselet MF . Animal models of Parkinson's disease progression. Acta Neuropathol 2008; 115: 385–398.
Teismann P, Vila M, Choi DK, Tieu K, Wu DC, Jackson-Lewis V et al. COX-2 and neurodegeneration in Parkinson's disease. Ann NY Acad Sci 2003; 991: 272–277.
Bove J, Perier C . Neurotoxin-based models of Parkinson's disease. Neuroscience 2012; 211: 51–76.
Fleming SM, Zhu C, Fernagut PO, Mehta A, DiCarlo CD, Seaman RL et al. Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp Neurol 2004; 187: 418–429.
Sedelis M, Schwarting RK, Huston JP . Behavioral phenotyping of the MPTP mouse model of Parkinson's disease. Behav Brain Res 2001; 125: 109–125.
Dick FD . Parkinson's disease and pesticide exposures. Br Med Bull 2006; 79-80: 219–231.
Perfeito R, Cunha-Oliveira T, Rego AC . Revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease—resemblance to the effect of amphetamine drugs of abuse. Free Radic Biol Med 2012; 53: 1791–1806.
Acknowledgements
This work was supported by grants from the National Research Foundation of Korea (2012R1A1A2007688 and 2012R1A2A2A01046822).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Hong, S., Hwang, J., Kim, J. et al. Heptachlor induced nigral dopaminergic neuronal loss and Parkinsonism-like movement deficits in mice. Exp Mol Med 46, e80 (2014). https://doi.org/10.1038/emm.2014.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2014.12
Keywords
This article is cited by
-
Environmental neurotoxic pollutants: review
Environmental Science and Pollution Research (2020)