Abstract
Glucagon-like peptide-1 (GLP-1) is a potent glucoincretin hormone and an important agent for the treatment of type 2 diabetes. Here we demonstrate that B-cell translocation gene 2 (BTG2) is a crucial regulator in GLP-1-induced insulin gene expression and insulin secretion via upregulation of pancreatic duodenal homeobox-1 (PDX-1) in pancreatic β-cells. GLP-1 treatment significantly increased BTG2, PDX-1 and insulin gene expression in pancreatic β-cells. Notably, adenovirus-mediated overexpression of BTG2 significantly elevated insulin secretion, as well as insulin and PDX-1 gene expression. Physical interaction studies showed that BTG2 is associated with increased PDX-1 occupancy on the insulin gene promoter via a direct interaction with PDX-1. Exendin-4 (Ex-4), a GLP-1 agonist, and GLP-1 in pancreatic β-cells increased insulin secretion through the BTG2–PDX-1–insulin pathway, which was blocked by endogenous BTG2 knockdown using a BTG2 small interfering RNA knockdown system. Finally, we revealed that Ex-4 and GLP-1 significantly elevated insulin secretion via upregulation of the BTG2–PDX-1 axis in pancreatic islets, and this phenomenon was abolished by endogenous BTG2 knockdown. Collectively, our current study provides a novel molecular mechanism by which GLP-1 positively regulates insulin gene expression via BTG2, suggesting that BTG2 has a key function in insulin secretion in pancreatic β-cells.
Similar content being viewed by others
Introduction
The postprandial enhancement of insulin secretion by hormonal factors from the gut is called the incretin effect.1, 2 The incretin hormone, glucagon-like peptide-1 (GLP-1), is produced from the gut endocrine L-cells in a nutrient-dependent manner and is also secreted in pancreatic islets.3 GLP-1 induces pancreatic β-cell neogenesis, proinsulin biosynthesis and glucose-stimulated insulin secretion, and it inhibits food intake, gastric emptying, acid secretion and glucagon secretion, both in normal subjects and in patients with type 2 diabetes.2, 4 The pancreatic β-cell growth-promoting action of GLP-1 acts as its insulinotropic activity through a G-protein-coupled receptor by interacting with a specific receptor, the GLP-1 receptor. In addition, GLP-1 regulates a diverse set of intracellular signals, such as cyclic-AMP-dependent protein kinase A, calcium and Epac in pancreatic β-cells.5, 6, 7 GLP-1 activates all the steps of insulin biosynthesis and gene expression, which enhances insulin secretion. Previous reports have shown that the transcriptional activity of the insulin gene is mainly mediated by a network of transcription factors, such as pancreatic duodenal homeobox-1 (PDX-1), β-cell E-box transcription factor (BETA2/NeuroD) and musculoaponeurotic fibrosarcoma oncogene family A (MafA), which are predominantly expressed in pancreatic β-cells.8, 9
B-cell translocation gene 2 (BTG2) is a member of the BTG/Tob gene family of antiproliferative genes that has been shown to be a crucial factor of the cell cycle machinery and its processes, such as cell differentiation, cell growth and survival.10, 11 BTG2 is predominantly expressed in the kidneys and lungs, but it has been detected in a variety of tissues, including liver, pancreas and intestine.12 The pleiotropic effect of BTG2 was initially reported to be induction of an immediate early gene by growth factors and tumor promoters, as the transcript and protein are detected very rapidly after phorbol ester or growth factor treatment in the various cell types.13, 14 Previous studies have shown that BTG2, a transcriptional coregulator, regulates the transcriptional activity of several gene promoters, such as Id3, cyclin D1 and RAR-β.11, 15, 16, 17 Most of all, a previous report has shown that pregnancy, known to cause an increment in the proliferation of pancreatic β-cells and insulin release, significantly upregulated the BTG2 gene expression in rodent pancreatic islets, suggesting that BTG2 may be involved in insulin secretion.18 However, the biological relevance of BTG2 in insulin gene expression and the possible physiological role have not been reported yet.
In this study we have demonstrated that GLP-1 increases insulin gene expression and insulin secretion via the induction of BTG2–PDX-1 axis in pancreatic β-cells, and that this stimulatory effect of GLP-1 was markedly reduced by endogenous knockdown of BTG2. Thus, we reveal a transcriptional coregulator BTG2 as a novel key factor in insulin gene expression and insulin secretion.
Materials and methods
Plasmids
The reporter plasmids for human (hINS-Luc) and rat II insulin promoter (rINS-Luc) were previously described.19, 20 Expression vectors for PDX-1, BTG2, pEBG vector (GST), and GST–BTG2 were described previously.21, 22 All plasmids were confirmed by sequencing analysis.
Cell culture and transient transfection assays
INS-1 (rat insulinoma cells) cells were cultured in RPMI 1640 (Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA), 2-mercaptoethanol (50 μM) and antibiotics in a humidified atmosphere containing 5% CO2 at 37 °C. Transient transfections were conducted as previously described.21, 23
Preparation of recombinant adenovirus and small interfering RNA experiments
Adenovirus encoding BTG2 (Ad-BTG2) and the small interfering RNAs (siRNAs) for BTG2 (si Scram and si BTG2) have been prepared according to the method described previously.22 To express the BTG2 and siRNA for BTG2, cells were infected with the indicated amounts of Ad-BTG2 and siRNA BTG2. Each of the samples was used for northern blot and western blot analysis. The sequences of siRNA are as follows: scrambled siRNA, 5′-ATGAGCCACGGGAAGAG-AACC-3′ and BTG2 siRNA, 5′-CTATCGCTTACCGCAT-CAA-3′.
Northern blot analysis
Total RNA was isolated from INS-1 cells and mouse primary islets using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. Briefly, aliquots of 30 μg of total RNA from each of the samples were used for northern blot analysis as previously described.21, 23 The probe labeling of each of the cDNAs for BTG2, PDX-1, MafA, insulin and glyceraldehyde 3-phosphate dehydrogenase with [α-32P]dCTP was performed with a random-primer DNA labeling system (Amersham Biosciences, Little Chalfont, UK). All transcripts were normalized with glyceraldehyde 3-phosphate dehydrogenase expression.
Quantitative PCR
Total RNA from INS-1 cells was extracted using an RNeasy minikit (Qiagen, Valencia, CA, USA). cDNA generation by First Strand cDNA synthesis kit (Fermentas, Burlington, ON, Canada) was performed by using the Power SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK) with the StepOnePlus Real-Time PCR (Applied Biosystems), as previously described.21, 24 All data were expressed as a ratio of the target gene to β-actin expression. The primer sequences used for PCR are as follows: BTG2, 5′-CCCCGGTGGCTGCCTCCTATG-3′ (forward) and 5′-GGGTCGGGTGGCTCCTATCTA-3′ (reverse); PDX-1, 5′-CCGCGTTCATCTCCCTTTC-3′ (forward) and 5′-TGCCC-ACTGGCTTTTCCA-3′ (reverse); insulin, 5′-TCTTCTACACACAGTCCCG-3′ (forward) and 5′-AGTGCCAAGGTCTGAAGATCCC-3′ (reverse); and β-actin, 5′-CCC-GCGAGTACAACCTTCT-3′ (forward) and 5′-CGTCATCCATGGCGAACT-3′ (reverse).
GST pull-down and in vivo interaction assay
GST pull-down assay and in vivo interaction assay were performed according to the method described previously.22, 25
Western blot analysis
INS-1 cells and mouse primary islets were isolated and processed according to a method described previously.21, 26 The membranes were probed with BTG2, PDX-1 and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and were then developed using an ECL western blot detection kit (Amersham Bioscience).
Chromatin immunoprecipitation assay
The chromatin immunoprecipitation assay was performed as described previously.22, 26 Twenty-four hours after transfection with BTG2 in INS-1 cells, the cells were treated with GLP-1 (10 nM). The cells were subsequently collected and the chromatin immunoprecipitation assay was performed with anti-PDX-1. The final DNA extractions were quantified by PCR with two pairs of primers for the proximal (−276/−56 bp) and distal (−461/−317 bp) regions of the insulin promoter. The specific primers used for PCR are as follows: proximal, 5′-GACATTTGCCCCCAGCTGTG-3′ (forward) and 5′-TCAACCCCTGCCGCCTGGCC-3′ (reverse); distal, 5′-GTCCTGGGGACAGGGGTCTG-3′ (forward) and 5′-TAGACCAGGAGAGCTGGAGG-3′ (reverse).
Animals
Eight-week-old, male C57BL/6 mice (Daehan Biolink, Chungbuk, Republic of Korea) were used for this experiment. For the exendin-4 (Ex-4) stimulation experiment, mice were injected intraperitoneally with Ex-4 (20 μg kg−1) for 7 days. The mice were killed and their pancreatic islets were isolated for performing western and northern blot analyses. All experimental procedures and protocols were approved and conducted in accordance with the regulations of the Institutional Animal Care and Use Committee of the Daegu Hanny University.
Islet isolation and measurement of insulin secretion
Mouse pancreatic islets were isolated from the pancreas of C57BL/6 mice by the collagenase digestion technique, and insulin secretion was performed according to the method previously described.21
Statistical analysis
Data are expressed as the mean (±s.e.m.). Analysis of variance was employed to determine significant differences as detected by Student’s t-tests and/or one-way analysis of variance methods. Statistical significance was considered at P<0.05.
Results
GLP-1 induces insulin gene expression via BTG2 induction in pancreatic β-cells
Previous studies have shown that GLP-1 stimulates insulin gene transcription via a diverse network of transcription factors, such as PDX-1, MafA and BETA2.8, 9, 27 On the basis of these findings, we examined the biological relevance of BTG2 in GLP-1-induced insulin gene expression in pancreatic β-cells. Treatment of INS-1 with GLP-1 significantly increased BTG2 mRNA expression in a time- and dose-dependent manner, and subsequently elevated PDX-1 and insulin mRNA levels in pancreatic β-cells (Figures 1a and b), consistent with the previous reports. Next we further confirmed whether GLP-1 also regulates the BTG2 protein levels in pancreatic β-cells. GLP-1 treatment significantly increased BTG2 and, subsequently, PDX-1 protein levels in a time-dependent manner (Figure 1c), which is consistent with the increase in BTG2 and PDX-1 mRNA expression. Overall, these results strongly suggest a novel crucial role of BTG2 in regulating GLP-1-mediated insulin gene expression.
GLP-1 induces the BTG2 gene expression. (a) INS-1 cells were treated with GLP-1 (10 nM) for various time periods up to 24 h. (b) INS-1 cells were treated with GLP-1 for 1 h at the indicated concentrations. The levels of BTG2, PDX-1 and insulin mRNA were measured by northern blot analysis and then normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (c) INS-1 cells were treated with GLP-1 (10 nM) for various time periods up to 24 h. Whole-cell extracts were isolated and analyzed using western blot analysis with the indicated antibodies. Protein level was normalized to β-actin.
BTG2 increases insulin gene expression and secretion via induction of PDX-1
As PDX-1 has a critical role in insulin gene expression and insulin production, we attempted to elucidate the potential link between BTG2 and PDX-1 in pancreatic β-cells. As shown in Figure 2a, overexpression of BTG2 using Ad-BTG2 significantly increased PDX-1 and insulin mRNA levels in a dose-dependent manner, but it did not significantly change MafA mRNA levels (Figure 2a). Next we further confirmed whether BTG2 also regulates transcriptional activity of human and rat insulin gene promoter in pancreatic β-cells. As expected, BTG2 significantly increased the transcriptional activity of insulin gene promoters in a dose-dependent manner (Figures 2b and c) and in a pattern similar to that indicated by insulin gene expression. Finally, we examined the potential effect of BTG2 on insulin secretion in pancreatic β-cells. GLP-1 significantly elevated insulin secretion from INS-1 cells. Interestingly, insulin secretion was increased by Ad-BTG2 relative to the control cells (Figure 2d). Taken together, these results show that BTG2 has a key function in insulin gene expression and secretion through PDX-1 induction in pancreatic β-cells.
BTG2 elevates insulin secretion. (a) INS-1 cells were infected with Ad-BTG2 at a multiplicity of infection (MOI) of 30 or 60 for 24 h. Total RNA was extracted from pancreatic β-cells and utilized for northern blot analysis. BTG2, PDX-1, MafA and insulin mRNA levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (b and c) INS-1 cells were transfected with BTG2 in the indicated reporter genes. Luciferase activity was measured after 36 h and normalized to β-galactosidase activity. (d) Insulin secretion was performed from INS-1 cells infected with Ad-GFP and Ad-BTG2 for 24 h at the indicated concentrations, using a radioimmunoassay kit. GLP-1 (10 nM) was used as a positive control. All data are representative of at least three independent experiments. *P<0.05 when compared with untreated control.
BTG2 physically interacts with PDX-1 and mediates PDX-1 occupancy on the insulin gene promoter
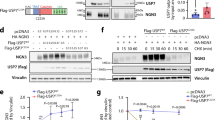
As in addition to PDX-1 abundance, PDX-1 binding to the insulin gene promoter is a major determinant of insulin gene expression and production, we elucidated the physical interaction between BTG2 and PDX-1 using in vitro and in vivo GST pull-down assays. The results of the GST pull-down assays demonstrated that labeled PDX-1 interacted with GST–BTG2 (Figure 3a) and, subsequently, PDX-1 bound to GST–BTG2, but not to GST alone (Figure 3b). Collectively, these results demonstrate that BTG2 physically interacts with PDX-1 in vitro and in vivo. To further confirm whether BTG2 and PDX-1 interaction affects PDX-1 occupancy on the insulin gene promoter, we performed the chromatin immunoprecipitation assay in pancreatic β-cells. The endogenous PDX-1 recruitment to the proximal region of the insulin gene promoter by GLP-1 treatment was significantly enhanced in BTG2-overexpressing cells when compared with the control cells (Figure 3c, top panel). However, the nonspecific distal region of the insulin gene promoter was unable to recruit this protein under all conditions (Figure 3c, bottom panel). Overall, these findings strongly suggest that BTG2 has a key function in PDX-1 occupancy on the insulin gene promoter via a physical interaction.
Interaction between BTG2 and PDX-1. (a) In vitro GST pull-down assay. PDX-1 protein was labeled with [35S] methionine by in vitro translation and incubated with bacterially expressed pEBG (GST alone) and pEBG–BTG2 (GST–BTG2) fusion protein. (b) In vivo interaction between BTG2 and PDX-1. INS-1 cells were cotransfected with expression vectors for PDX-1 along with GST–BTG2 and pEBG (GST alone) as a control. The complex formed (top panel) and the amount of PDX-1 used for the in vivo binding assay (bottom panel, lysate) was analyzed by western blot analysis using anti-PDX-1 antibody. The same blot was stripped and reprobed with an anti-GST antibody (middle panel) to confirm the expression levels of GST–BTG2 and the GST control. (c) Chromatin immunoprecipitation assay shows PDX-1 occupancy on the insulin gene promoter. INS-1 cells were transfected with BTG2 for 24 h and then treated with GLP-1 (10 nM) for 1 h. Soluble chromatin was immunoprecipitated with anti-PDX-1 antibody or IgG as indicated. Purified DNA samples were employed for PCR with primers binding to the specific proximal (top panel) and nonspecific distal (bottom panel) regions of the insulin gene promoter. Ten percent of the soluble chromatin was used as an input.
Ex-4-induced insulin secretion is mediated by BTG2
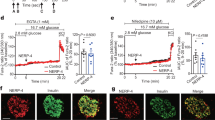
To confirm the transduction efficiency of BTG2 siRNA, we performed western blot analysis on pancreatic β-cell extracts. As expected, successful transduction of endogenous BTG2 siRNA was confirmed by western blot analysis conducted with the indicated antibodies in INS-1 cells (Figure 4a). Moreover, we determined the crucial role of BTG2 in the regulation of insulin and PDX-1 gene expression following GLP-1 treatment. Ex-4, a GLP-1 agonist, and GLP-1 significantly increased BTG2, PDX-1 and insulin mRNA levels, and this stimulatory effect of GLP-1 was abolished by endogenous BTG2 knockdown using BTG2 siRNA. Overall, these results show that BTG2 has a critical function in the regulation of GLP-1-induced insulin gene expression. To investigate whether Ex-4-mediated insulin secretion is altered by BTG2, we performed a knockdown of endogenous BTG2 using BTG2 siRNA in INS-1 cells. As shown in Figures 4c and d, insulin secretion was significantly elevated upon Ex-4 treatment, in a manner similar to that of the elevation of insulin gene expression (Figure 4b), whereas this stimulatory effect of GLP-1 and/or Ex-4 was markedly reduced by endogenous BTG2 knockdown. Overall, these results strongly suggest that the stimulatory effect of GLP-1 on insulin secretion was altered by BTG2.
GLP-1-stimulated insulin secretion is mediated by BTG2. (a) INS-1 cells were transfected with scrambled siRNA or BTG2 siRNA for 36 h. Whole-cell extracts were isolated and analyzed using western blot analysis with the indicated antibodies. (b) INS-1 cells were transfected with scrambled siRNA and BTG2 siRNA for 36 h, and then treated with or without GLP-1 (10 nM) and Ex-4 (100 nM). Total RNA was isolated and analyzed using quantitative PCR analysis with observed primers. (c and d) INS-1 cells were transfected with BTG2 siRNA (si BTG2) and Scrambled siRNA (si Scram) for 36 h. After knockdown for 36 h, insulin secretion was performed from INS-1 cells treated with or without GLP-1 (10 nM) and Ex-4 treatment (100 nM) for 1−6 h using a radioimmunoassay kit. *P<0.05 and **P<0.05 compared with untreated control and GLP-1- and Ex-4-treated cells.
Ex-4 induces insulin gene expression via BTG2 induction in pancreatic islets
Previous studies have shown that GLP-1 and Ex-4, a GLP-1 agonist, elevate insulin gene expression and subsequently increase insulin secretion in rodent and human subjects.4, 8, 28, 29 On the basis of these findings, we confirmed the biological relevance between Ex-4-mediated insulin gene expression and BTG2 in animal experiments. In agreement with in vitro experiments, Ex-4 treatment significantly increased protein production and mRNA levels of BTG2 and PDX-1, and subsequently elevated insulin gene expression in pancreatic islets (Figures 5a and b). Taken together, these findings suggest that Ex-4 has an important function in the regulation of the PDX-1–insulin pathway via BTG2 induction in pancreatic islets. Finally, we confirmed the crucial role of BTG2 on Ex-4- and GLP-1-stimulated insulin secretion in pancreatic islets. As expected, Ex-4 and GLP-1 significantly elevated insulin secretion, consistent with the previous results (Figures 4c and d), and this stimulation was markedly abolished by BTG2 knockdown (Figure 5c). Overall, these results suggest that BTG2 has an important role in the positive regulation of Ex-4-stimulated insulin secretion in pancreatic islets.
Ex-4 increases insulin gene expression via BTG2 induction. (a and b) C57BL/6 mice were injected intraperitoneally with Ex-4 (20 μg kg−1 of body weight) for 7 days, and pancreatic islets were obtained for protein extracts to perform western blot analysis with various antibodies (a) and total RNA isolation for northern blot analysis using BTG2, PDX-1 and insulin probes (b). Protein and mRNA expression were normalized to β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) level, respectively. (c) Pancreatic islets were transfected with BTG2 siRNA (si BTG2) and Scrambled siRNA (si Scram) for 36 h. After knockdown for 36 h, insulin secretion was performed from pancreatic islets and treated with or without GLP-1 (10 nM) and Ex-4 (100 nM) using a radioimmunoassay kit. All mice were separated into experimental groups (n=4–6 mice per group). *P<0.05 and **P<0.05 compared with untreated control and GLP-1- and Ex-4-treated cells.
Discussion
In this study we have demonstrated that GLP-1 significantly elevated the BTG2 gene expression and that the overexpression of BTG2 using an adenoviral system (Ad-BTG2)-upregulated insulin gene expression and secretion via PDX-1 induction. Finally, this stimulatory effect of GLP-1 on insulin gene expression and secretion under both in vitro and in vivo conditions was effectively abolished by endogenous BTG2 knockdown. On the basis of these findings, we propose that the GLP-1–BTG2–PDX-1 network may provide a novel molecular mechanism in controlling insulin gene expression and may serve as a crucial factor in regulating insulin secretion.
A recent report suggests that pregnancy, known to cause an increment in the proliferation of pancreatic β-cells and insulin release, significantly upregulated the BTG2 gene expression in rodent pancreatic islets, suggesting that BTG2 may be involved in insulin secretion.18 However, there is no evidence showing a strong link between the GLP-1-mediated regulation of the BTG2 gene expression and its role in insulin secretion. Here we reveal that induction of BTG2 by GLP-1 mediates insulin release by upregulating PDX-1 via two major findings: First, we have verified a critical role of BTG2 in the GLP-1-dependent pathway in pancreatic β-cells. As expected, GLP-1 significantly elevated the BTG2, PDX-1 and subsequent insulin gene expression in pancreatic β-cells (Figure 1), and this phenomenon was abrogated by endogenous BTG2 knockdown (Figure 4). Second, overexpression of BTG2 significantly increased insulin secretion via the specificity of PDX-1 induction, in a manner similar to that observed by GLP-1 treatment in pancreatic β-cells (Figure 1). Collectively, our current findings suggest that BTG2 has a crucial function in controlling GLP-1-stimulated insulin release by PDX-1 induction.
As mentioned previously, PDX-1 acts as a member of transcriptional factors, such as MafA and BETA2/NeuroD, and subsequently enhances insulin gene expression.8, 9, 21, 27 Indeed, we observed that BTG2, a transcriptional cofactor, downregulated the promoters of Id3 and cyclin D1, and upregulated the RAR-β transcriptional activity.11, 15, 16, 17 These findings suggest that BTG2 may have some function in controlling both insulin gene expression and insulin release; however, the biological relationship between BTG2 and several transcription factors with respect to regulating insulin gene expression and insulin release has yet to be fully elucidated. In the present study we elucidated a novel transcriptional coregulator responsive to insulin gene expression following induction of the GLP-1–BTG2–PDX-1 network under in vitro and in vivo conditions, such as gain- and loss-of-function experiments, physical interaction assays and GLP-1 agonist injection (Figures 2, 3, 4). Overall, these findings suggest that BTG2 is a crucial cofactor in controlling insulin secretion and/or production in pancreatic β-cells. However, we cannot rule out the possibility that the detailed molecular mechanism of a link between the GLP-1–BTG2/PDX-1 network and insulin gene transcription may also rely on other unknown transcription factors that regulate the stimulation pattern of insulin gene expression.
Previous reports from our group demonstrated that the induction of the BTG2 gene expression by the glucagon–CREB-dependent pathway led to increase of glucose production, which was abolished by insulin treatment in hepatocytes.22 Moreover, Cui et al. 30 showed that growth hormone, which is a stimulator of hepatic gluconeogenesis, significantly increases BTG2 via the C/EBP-CREB pathway in adipocytes, suggesting that BTG2 may be involved in hepatic gluconeogenesis, consistent with a previous report. A recent report suggests that liraglutide, a GLP-1 agonist, increases the ATP/AMP ratio, which in turn inhibits AMP-activated protein kinase. Inhibition of the AMP-activated protein kinase by liraglutide prevents pancreatic β-cell glucolipotoxicity by activating the mammalian target of rapamycin and its downstream effectors P70S6K and 4E-BP1, thereby promoting pancreatic β-cell proliferation and apoptosis.31 Interestingly, our current findings demonstrate that GLP-1 increases insulin gene expression and insulin secretion via the induction of BTG2–PDX-1 axis in pancreatic β-cells, and this stimulatory effect of GLP-1 was abolished by endogenous knockdown of BTG2. On the basis of these findings, our current study provides a novel insight into the mechanism by which BTG2 affects insulin secretion, following the upregulation of the PDX-1-dependent pathway. However, we cannot rule out the possibility that BTG2 may also rely on unknown mechanisms of protein stability, degradation, other unknown transcription factors and other signaling pathways to regulate the induction of insulin gene expression. Therefore, a further detailed explanation of the GLP-1–BTG2 axis and insulin secretion is required in an animal model, such as BTG2-null mice and an insulin-resistant mouse model in the future.
In conclusion, our current study demonstrates that BTG2 acts as a novel cofactor to regulate insulin gene transcription and/or secretion. These results suggest that the induction of BTG2 by GLP-1 upregulates insulin release via PDX-1 induction both in vitro and in vivo. We suggest that BTG2 has another pleiotropic effect on insulin secretion in pancreatic β-cells. Therefore, a novel molecular mechanism involved in PDX-1 induction by BTG2 may provide new insights into the beneficial role of insulin resistance and development of novel therapeutic agents to treat metabolic diseases.
References
Kjems LL, Holst JJ, Vølund A, Madsbad S . The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 2003; 52: 380–386.
Meier JJ, Gallwitz B, Schmidt WE, Nauck MA . Glucagon-like peptide 1 as a regulator of food intake and body weight: therapeutic perspectives. Eur J Pharmacol 2002; 440: 269–279.
D’Alessio DA, Vahl TP . Glucagon-like peptide 1: evolution of an incretin into a treatment for diabetes. Am J Physiol Endocrinol Metab 2004; 286: E882–E890.
Deacon CF . Therapeutic strategies based on glucagon-like peptide 1. Diabetes 2004; 53: 2181–2189.
Perry T, Greig NH . The glucagon-like peptides: a double-edged therapeutic sword? Trends Phamacol Sci 2003; 24: 377–383.
Holz GG . Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes 2004; 53: 5–13.
Yu Z, Jin T . New insights into the role of cAMP in the production and function of the incretin hormone glucagon-like peptide-1 (GLP-1). Cell Signal 2010; 22: 1–8.
Glick E, Leshkowitz D, Walker MD . Transcription factor BETA2 acts cooperatively with E2A and PDX-1 to activate the insulin gene promoter. J Biol Chem 2000; 275: 2199–2204.
Ye DZ, Tai MH, Linning KD, Szabo C, Olson LK . MafA expression and insulin promoter activity are induced by nicotinamide and related compounds in INS-1 pancreatic beta-cells. Diabetes 2006; 55: 742–750.
Tirone F . The gene PC3(TIS21/BTG2), prototype member of the PC3/BTG/TOB family: regulator in control of cell growth, differentiation, and DNA repair? J Cell Physiol 2001; 187: 155–1165.
Lim IK . TIS/21 (BTG2/PC3) as a link between ageing and cancer: cell cycle regulator and endogenous cell death molecule. J Cancer Res Clin Oncol 2006; 132: 417–426.
Melamed J, Kernizan S, Walden PD . Expression of B-cell translocation gene 2 protein in normal human tissues. Tissue Cell 2002; 34: 28–32.
Bradbury A, Possenti R, Shooter EM, Tirone F . Molecular cloning of PC3, a putatively secreted protein whose mRNA is induced by nerve growth factor and depolarization. Proc Natl Acad Sci USA 1991; 88: 3353–3357.
Varnum BC, Reddy ST, Koski RA, Herschman HR . Synthesis, degradation, and subcellular localization of proteins encoded by the primary response genes TIS/PC4 and TIS21/PC3. J Cell Physiol 1994; 158: 205–213.
Farioli-Vecchioli S, Tanori M, Micheli L, Mancuso M, Leonardi L, Saran A et al. Inhibition of medulloblastoma tumorigenesis by the antiproliferative and pro-differentiative gene PC3. FASEB J 2007; 21: 2215–2225.
Passeri D, Marcucci A, Rizzo G, Billi M, Panigada M, Leonardi L et al. Btg2 enhance retinoic acid-induced differentiation by modulating histone H4 methylation and acetylation. Mol Cell Biol 2006; 26: 5023–5032.
Farioli-Vecchioli S, Saraulli D, Costanzi M, Leonardi L, Cinà I, Micheli L et al. Impaired terminal differentiation of hippocampal granule neurons and defective contextual memory in PC3/Tis21 knockout mice. PLoS One 2009; 17: e8339.
Chan G, Lin C, Xue Y, Mao X, Xu K, Liu C . Molecular mechanism of pancreatic β-cell adaptive proliferation: studies during pregnancy in rats and in vitro. Endocrine 2011; 39: 118–127.
Jang WG, Kim EJ, Park KG, Park YB, Choi HS, Kim HJ et al. Glucocorticoid receptor mediated repression of human insulin gene expression is regulated by PGC-1alpha. Biochem Biophys Res Commun 2007; 352: 716–721.
Fox N, Schrementi J, Nishi M, Ohagi S, Chan SJ, Heisserman JA et al. Human islet amyloid polypeptide transgenic mice as a model of non-insulin-dependent diabetes mellitus (NIDDM). FEBS Lett 1993; 323: 40–44.
Park KG, Lee KM, Seo HY, Suh JH, Kim HS, Wang L et al. Glucotoxicity in the INS-1 rat insulinoma cell line is mediated by the orphan nuclear receptor small heterodimer partner. Diabetes 2007; 56: 431–437.
Hwang SL, Kwon O, Lee SJ, Kim YD, Choi JH . B-cell translocation gene-2 increases hepatic gluconeogenesis via induction of CREB. Biochem Biophys Res Commun 2012; 427: 801–805.
Kim YD, Park KG, Lee YS, Park YY, Kim DK, Nedumaran B et al. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 2008; 57: 306–314.
Hagman DK, Hays LB, Parazzoli SD, Poitout V . Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J Biol Chem 2005; 280: 32413–32418.
Lee JM, Seo WY, Song KH, Chanda D, Kim YD, Kim DK et al. AMPK-dependent repression of hepatic gluconeogensis via disruption of CREB.CRTC2 complex by orphan nuclear receptor small heterodimer partner. J Biol Chem 2010; 285: 32182–32191.
Kim JY, Kim HJ, Kim KT, Kim YY, Seong HA, Park KC et al. Orphan nuclear receptor small heterodimer partner represses hepatocyte nuclear factor 3/Foxa transactivation via inhibition of its DNA binding. Mol Endocrinol 2004; 18: 2880–2894.
Melloul D, Marshak S, Cerasi E . Regulation of insulin gene transcription. Diabetologia 2002; 45: 309–326.
Alarcon C, Wicksteed B, Rhodes CJ . Exendin-4 controls insulin production in rat islet beta cell predominantly by potentiation of glucose-stimulated proinsulin biosynthesis at the translational level. Diabetologia 2006; 49: 2920–2929.
Egan JM, Clocquet AR, Elahi D . The insulinotropic effect of acute exendin-4 administered to humans: comparison of nondiabetic state to type 2 diabetes. J Clin Endocrinol Metab 2002; 87: 1282–1290.
Cui TX, Lin G, LaPensee CR, Calinescu AA, Rathore M, Streeter C et al. C/EBPβ mediates growth hormone-regulated expression of multiple target genes. Mol Endocrinol 2011; 25: 681–693.
Miao XY, Gu ZY, Liu P, Hu Y, Li L, Gong YP et al. The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides 2013; 39: 71–79.
Acknowledgements
We thank Dr Keun-Gyu Park (Kyungpook National University School of Medicine, Daegu, Republic of Korea) for his critical suggestions and helpful discussions. We also wish to thank other lab members for their helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Hwang, SL., Kwon, O., Kim, SG. et al. B-cell translocation gene 2 positively regulates GLP-1-stimulated insulin secretion via induction of PDX-1 in pancreatic β-cells. Exp Mol Med 45, e25 (2013). https://doi.org/10.1038/emm.2013.47
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2013.47
Keywords
This article is cited by
-
Enhancement of the SESN2-SHP cascade by melatonin ameliorates hepatic gluconeogenesis by inhibiting the CRBN-BTG2-CREBH signaling pathway
Experimental & Molecular Medicine (2023)
-
B-cell translocation gene 2 enhances fibroblast growth factor 21 production by inducing Kruppel-like factor 15
Scientific Reports (2019)