Abstract
Persistently activated JAK/STAT3 signaling pathway plays a pivotal role in various human cancers including major carcinomas and hematologic tumors, and is implicated in cancer cell survival and proliferation. Therefore, inhibition of JAK/STAT3 signaling may be a clinical application in cancer therapy. Here, we report that 2-cyclohexylimino-6-methyl-6,7-dihydro-5H-benzo [1,3]oxathiol-4-one (BOT-4-one), a small molecule inhibitor of JAK/STAT3 signaling, induces apoptosis through inhibition of STAT3 activation. BOT-4-one suppressed cytokine (upd)-induced tyrosine phosphorylation and transcriptional activity of STAT92E, the sole Drosophila STAT homolog. Consequently, BOT-4-one significantly inhibited STAT3 tyrosine phosphorylation and expression of STAT3 downstream target gene SOCS3 in various human cancer cell lines, and its effect was more potent in JAK3-activated Hodgkin's lymphoma cell line than in JAK2-activated breast cancer and prostate cancer cell lines. In addition, BOT-4-one-treated Hodgkin's lymphoma cells showed decreased cell survival and proliferation by inducing apoptosis through down-regulation of STAT3 downstream target anti-apoptotic gene expression. These results suggest that BOT-4-one is a novel small molecule inhibitor of JAK3/STAT3 signaling and may have therapeutic potential in the treatment of human cancers harboring aberrant JAK3/STAT3 signaling, specifically Hodgkin's lymphoma.
Similar content being viewed by others
Introduction
The JAK/STAT signaling cascade was originally characterized during interferon-mediated signal transduction studies in early 1990s (Schindler et al., 1992; Shuai et al., 1992; Müller et al., 1993; Watling et al., 1993). JAKs belong to a family of non-receptor tyrosine kinases and STATs are latent cytosolic transcription factors that activate signals from the cell membrane to the nucleus. The JAK and STAT protein families are composed of four and seven members in mammals, respectively and JAK/STAT pathways are up-regulated by more than fifty different cytokines and growth factors (Schindler and Plumlee, 2008). The binding of cytokines and growth factors to their corresponding transmembrane receptors subsequently activates membrane-associated JAK and STAT proteins by phosphorylation of specific tyrosine residues. STAT proteins are a family of cytosolic transcription factors that have dual functions - they transduce signals through the cytoplasm and have a function as transcription factors in the nucleus (Takeda and Akira, 2000).
The JAK/STAT-mediated signaling cascade represents essential roles for proliferation or differentiation, development, hematopoiesis, and immune responses (Park et al., 1995; Meraz et al., 1996; Darnell, 1997; Neubauer et al., 1998). However, recent studies showed that persistently activated JAK/STAT signaling correlates with tumorigenesis and cancer progression through its intimate connection to growth factor signaling and observed high frequency in human cancers. Numerous studies have shown that constitutively activated JAK kinases are found in a variety of cancer patients with lymphoblastic leukemia, myeloproliferative diseases, acute megakaryoblastic leukemia, and acute lymphoblastic leukemia (James et al., 2005; Walters et al., 2006; Bercovich et al., 2008; Flex et al., 2008; Mullighan et al., 2009; Oh et al., 2010). In addition, STAT3, in part STAT5 and STAT6, is also constitutively activated in multiple human cancers as well as in various hematopoietic malignancies (Klampfer, 2006; Yu et al., 2009; Haftchenary et al., 2011). Therefore, regulation of inappropriately activated JAK and/or STAT signaling is valuable therapeutic targets for the treatment of human cancers. Several JAK/STAT inhibitors have been developed and are used on clinical trials for the cancer treatments (O'Shea et al., 2004; Atallah and Verstovsek, 2009; Fletcher et al., 2009; Haftchenary et al., 2011).
Benzoxathiol derivatives, especially 6-hydroxy-1,3-benzoxathol-2-one (called also tioxolone) have been used in the local therapy of psoriasis vulgaris and acne, and also reported to have anti-bacterial, anti-mycotic, and cytostatic properties (Goeth and Wildfeuer, 1969; Wildfeuer, 1970; Lius and Sennerfeldt, 1979). Recent report showed that benzoxathiol derivativs have anti-inflammatory and anti-tumorigenic effects through inhibition of NF-κB and STAT3 activation (Kim et al., 2008a, 2008c). We herein identified 2-cyclohexylimino-6-methyl-6,7-dihydro-5H-benzo[1,3]oxathiol-4-one (BOT-4-one) has a potent anti-cancer activity via inhibition of JAK/STAT3 signaling in both Drosophila and human cancer cells. BOT-4-one inhibited persistently activated cancer cell proliferation and survival through induction of apoptosis by down-regulation of antiapoptotic gene expressions, which are known to STAT3 downstream target molecules. BOT-4-one predominantly induced cell death in Hodgkin's lymphoma L540 cells that are aberrantly activated JAK3/STAT3 signaling.
Results
BOT-4-one inhibits STAT92E activation in Drosophila cells
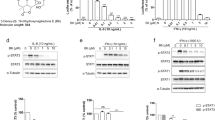
Drosophila cells have one JAK and one STAT protein called Hop and STAT92E compared with those of mammalian cells, respectively (Hou and Perrimon, 1997). To identify small molecules that are potential inhibitors of JAK/STAT signaling, we performed a cell-based high throughput screening using Drosophila cell line as previously described (Kim et al., 2008b, 2010a) and identified 2-cyclohexylimino-6-methyl-6,7-dihydro-5H-benzo[1,3]oxa thiol-4-one (BOT-4-one; Figure 1A) as a potential inhibitor of STAT92E signaling. Cytokine (upd)-induced STAT92E transcriptional activity was increased more than 21-fold compared to that of vehicle treatment and BOT-4-one was found to inhibit STAT92E transcriptional activity in a dose-dependent manner (Figure 1B). Cytokine-induced phosphorylation of tyrosine residues is a key step in STAT activation. To determine whether BOT-4-one could affect tyrosine phosphorylation, we examined tyrosine phosphorylation levels of STAT92E followed cytokine treatment. Treatment with 30 µM BOT-4-one almost completely suppressed STAT92E phosphorylation (Figure 1C). These results indicate that BOT-4-one is a small molecule inhibitor of STAT92E signaling in Drosophila cells.
BOT-4-one inhibits phosphorylation and transcription of STAT92E in Drosophila cells. (A) The chemical structure of BOT-4-one (C14H19NO2S; M.W., 265.4). (B) BOT-4-one inhibited cytokine (upd)-induced tyrosine phosphorylation of STAT92E. S2-NP cells transiently transfected with an expression plasmid for STAT92E-HA were co-cultured with upd-producing cells for 24 h in the presence of either vehicle (DMSO) alone or BOT-4-one. Immunoblot analysis was performed with phospho-STAT92E and HA antibodies. STAT92E-HA served as a loading control. (C) BOT-4-one inhibited STAT92E transcriptional activity. Cultured Drosophila S2-NP-STAT92E cells expressing a STAT92E reporter gene were co-cultured with upd-producing cells for 24 h in the presence of BOT-4-one. The STAT92E luciferase activity was measured and the firefly luciferase activity was normalized to Renilla luciferase activity. Results are shown as the mean of three independent experiments (± SD indicated by error bar). *P < 0.001, significant difference when the value of treatment was compared to that of the control.
BOT-4-one inhibits STAT3 activation in human cancer cell lines
We next examined the effect of BOT-4-one on the expression levels of STATs in various human cancer cell lines. Treatment with 30 µM BOT-4-one showed reduction of STAT3 expression in all cancer cell lines, and the effect was much stronger in L540 cells compared to MDA-MB-468 and DU145 cells (Figure 2A). STAT1 and STAT5 expression levels were also decreased in L540 cells by BOT-4-one, however, their expressions were not affected in MDA-MB-468 and DU145 cells. We therefore examined the dose effect of BOT-4-one on STATs phosphorylation in L540 cells. BOT-4-one significantly decreased STAT3 and STAT5 phosphorylation compared to that of STAT1 (Figure 2B), indicating that BOT-4-one selectively inhibits STAT3 and STAT5 phosphorylation in L540 cells. Phosphorylated STAT proteins on tyrosine residues undergo dimerization and translocation to the nucleus, where they initiate transcription and translocated STAT3 proteins to the nucleus undergoes dephophorylation and exports to the cytosolic region through the nuclear pore complex by nucleocytoplasmic shuttling (Herrmann et al., 2007). We next examined whether BOT-4-one could reduce tyrosine-phosphorylation status of STAT3. BOT-4-one inhibits phosphorylation of STAT3 in the cytosolic and nucleic regions, but its expression is not altered in the both regions (Figure 2C). In L540 cells, JAK3 is constitutively activated and JAK family kinases are upstream regulator of STATs activation. These results therefore suggest that BOT-4-one inhibits STAT3 activation, but not STAT3 expression and BOT-4-one may suppose the inhibition of JAK3 activity in L540 cells.
BOT-4-one inhibits expression and phosphorylation of STAT3 in human cancer cell lines. (A) Human cancer cell lines that express constitutively-active STAT3 were incubated with either vehicle (DMSO) alone or BOT-4-one (30 µM) for 24 h. Total RNA was extracted and subjected to quantitative real-time PCR. BOT-4-one inhibited STAT3 expression in all cell lines, but inhibition of STAT1 and STAT5 expression was cell type-dependent manner. Results are shown as the mean of three independent experiments (± SD indicated by error bar). *P < 0.001, significant difference when the value of treatment was compared to that of the control. (B) Whole cell extracts were prepared from L540 cells after treatment for 24 h with either vehicle (DMSO) alone or BOT-4-one, and immunoblot analysis was performed with antibodies specific for the molecules indicated. BOT-4-one inhibited tyrosine phosphorylation of STATs, and its inhibitory effect on STAT3 and STAT5 was much higher than those of STAT1. GAPDH served as a loading control. (C) Cytosolic and nucleic fractions were extracted from L540 cells after treatment for 24 h with either vehicle (DMSO) alone or BOT-4-one, and immunoblot analysis was performed with phospho-STAT3 and STAT3 antibodies. BOT-4-one inhibited STAT3 activation. C, cytosolic, N, nucleic.
BOT-4-one predominantly inhibits JAK3/STAT3 signaling
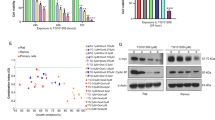
L540 cells are persistently activated JAK3/STAT3 pathway, whereas MDA-MB-468 and DU145 cells are persistently activated JAK1/STAT3 and JAK2/STAT3 pathways (Kim et al., 2010b). BOT-4-one strongly decreased STAT3 phosphorylation, in part STAT5, in L540 cells than in MDA-MB-468 and DU145 cells. In order to identify the effect of BOT-4-one on specificity of JAK3, we examined the effect of BOT-4-one on phosphorylation of JAK2, JAK3 and Src family kinases as well as ERK signaling. Reduction of JAK3/STAT3 activation by BOT-4-one in L540 cells was stronger than that of JAK2/STAT3-activated MDA-MB-468 and DU145 cells (Figures 3A-C). In addition, expression of the STAT3 target protein SOCS3 also inhibited and the effect was parallel as JAK/STAT3 inhibition in the cells. However, phosphorylation of Src family tyrosine kinases such as Lyn and Src was weakly affected upon 30 µM BOT-4-one in all cell lines and ERK phosphorylation was inhibited only in MDA-MB-468 and DU145 cells, but not in L540 cells. These results suggest that BOT-4-one inhibits STAT3 activation through a little different pathways in various cancer cell lines.
BOT-4-one inhibits JAK/STAT3 signaling. L540 (A), MDA-MB-468 (B), and DU145 (C) cells were incubated with either vehicle (DMSO) alone or BOT-4-one for 24 h. Whole cell extracts were proceeded for immunoblot analysis using antibodies specific for the molecules indicated. BOT-4-one inhibited constitutively-active STAT3 in all cell lines. Interestingly, BOT-4-one predominantly inhibited JAK3-mediated STAT3 phosphorylation and SOCS3 expression known to STAT3 downstream target molecule than those of JAK2-mediated. However, BOT-4-one inhibited ERK1/2 phosphorylation in a cell type-dependent manner, and has weak effect on Src family kinases such as Src and Lyn in all cell lines. GAPDH served as a loading control.
BOT-4-one inhibits cancer cell survival
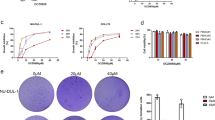
A number of studies reported that inhibition of STAT3 signaling reduce cancer cell survival (Al Zaid Siddiquee and Turkson, 2008). We next examined whether BOT-4-one reduces cancer cell survival by down-regulation of STAT3 activation. For the assay, L540 or DG-75 cells were treated with either vehicle alone or various concentrations of BOT-4-one. We found that viability and proliferation of L540 cells were significantly decreased by BOT-4-one in a dose- and time-dependent manner (Figures 4A and C). However, viability and proliferation of DG-75 cells were not affected by BOT-4-one, where STAT3 pathway was not activated (Kim et al., 2008b). IL-6 activates JAK/STAT signaling pathway by binding with IL-6R/gp130, and increases cancer cell survival and proliferation. To know that BOT-4-one could affect exogenous cytokine-induced cancer cell survival, we cultured L540 cells with IL-6 and measured cell viability. BOT-4-one inhibited IL-6 indued cancer cell survival in a dose-dependent manner and the effect was a little weaker than without IL-6 treatment (Supplementary Figure S1). Together, these results suggest that BOT-4-one affects cancer cell survival by downregulation of JAK/STAT3 signaling.
BOT-4-one affects cancer cell survival and proliferation. L540 (A and C) and DG-75 (B and D) cells were treated with either vehicle (DMSO) alone or BOT-4-one and incubated for the indicated time periods. Total and viable cell numbers were counted by trypan blue exclusion assay. The cell viability and proliferation of L540 cells were decreased by BOT-4-one in a dose- and time-dependent manner, but DG-75 cells were not affected, where JAK/STAT3 signaling was not activated. Results are shown as the mean of three independent experiments (± SD indicated by error bar). *P < 0.001; **P < 0.05, significant difference when the value of treatment was compared to that of the control.
BOT-4-one induces apoptosis through down-regulation of anti-apoptotic gene expression
To identify whether the inhibition of cell survival in BOT-4-one-treated L540 cells resulted in induction of apoptosis, we performed a TUNEL assay. Treatment with BOT-4-one increased TUNEL-positive cells about 6-fold compared to that of control cells (Figure 5A). Cleavage of Poly (ADP-ribose) polymerase (PARP) and caspase-3 are known to hallmarks of apoptosis (Decker et al., 2000). BOT-4-one increased cleaved fragments of both PARP and caspase-3 in a dose-dependent manner (Figure 5B). The inhibition of STAT3 signaling also reported to induce apoptosis by down-regulation of antiapoptotic gene expression (Iwamaru et al., 2007; Al Zaid Siddiquee and Turkson, 2008). To elucidate the molecular mechanism of BOT-4-one on apoptosis, we examined the expression levels of anti-apoptotic proteins known to STAT3 targets. BOT-4-one decreased the expression levels of anti-apoptotic mRNA and proteins such as Bcl-2, Bcl-xL, Mcl-1, and survivin in a dose-dependent manner (Figures 5C-E). These results indicate that BOT-4-one decreases cancer cell survival by inducing apoptosis through down-regulation of anti-apoptotic genes.
BOT-4-one induces apoptosis by down-regulation of anti-apoptotic gene expression. (A) L540 cells treated with either vehicle (DMSO) alone or BOT-4-one (30 µM) for 48 h were harvested, stained with fluorescein isothiocyanate-conjugated BrdU antibody and propidium iodide (PI). The cells were subsequently subjected to flow cytometry. A representative flow cytometric analysis was shown and the percentages of TUNEL-positive cells were indicated. (B and E) L540 cells were treated with either vehicle (DMSO) alone or BOT-4-one for 48 h, whole cell extracts were prepared and immunoblot analysis was performed with antibodies specific for the molecules indicated. (C and D) Total RNA was extracted from L540 cells treated with BOT-4-one for 8 h, and real-time PCR was performed. Anti-apoptotic gene expression was represented with relative fold (C) or stained with EtBr after electrophoresis (D). Treatment with BOT-4-one increased cleaved fragments of PARP and caspase-3, an apoptotic hallmarks (arrows in B), and decreased the expression of anti-apoptotic genes such as Bcl-2, Bcl-xL, Mcl-1, and survivin (C-E). GAPDH served as a loading control.
Discussion
Although benzoxathiol derivatives have been used in the treatment of psoriasis and acne, and reported to have anti-bacterial and cytostatic properties (Goeth and Wildfeuer, 1969; Wildfeuer, 1970; Lius and Sennerfeldt, 1979), the molecular basis of the pharmacological properties has not been defined yet. Psoriasis and acne are common inflammatory skin diseases that involved in immune responses. Recent reports showed that an anti-inflammatory effect of benzoxathiol derivatives was due to inhibition of NF-κB activation by targeting IKK as well as inhibition of STAT1 phosphorylation (Kim et al., 2008a, 2008d; Chung et al., 2009). NF-κB is one of the transcription factors implicated in inflammatory diseases. Therefore, blocking of NF-κB activation by benzoxathiol derivatives for the treatment of psoriasis was expected. JAK/STAT3 signaling is also activated in psoriasis and inhibition of this signaling may have therapeutic target for the treatment of the diseases (Chang et al., 2009; Miyoshi et al., 2011).
Persistent activation of JAK/STAT signaling, especially JAK/STAT3, is observed in various types of human cancers and contributes to tumorigenesis and cancer progression. The activation of STAT3 proteins in cancers is implicated to phosphorylation of JAK and Src family kinases (Niu et al., 2002; Klampfer, 2006; Yu et al., 2009; Hazan-Halevy et al., 2010). Accumulated results imply that development of new drugs to regulation of constitutively activated JAK/STAT3 is valuable therapeutic targets for cancer treatment. We found small molecule BOT-4-one as a potential inhibitor of JAK/STAT signaling using a cell-based high throughput screening in Drosophila cell line (Figure 1). The fruit fly Drosophila consists of only one JAK and one STAT (Hou and Perrimon, 1997). Despite the simplicity of the Drosophila JAK/STAT pathway, the mode of action of the JAK/STAT pathway in Drosophila is similar to that of mammals (Bach et al., 2003). Therefore, Drosophila to identify small molecule inhibitors of JAK/STAT signaling can serve as an excellent model organism (Arbouzova and Zeidler, 2006). BOT-4-one effectively inhibited cytokine-induced transcriptional activity and phosphorylation of STAT92E in Drosophila cells (Figures 1B and C).
Our previous results showed that identified small molecule inhibitors of STAT92E activity using Drosophila model were well-fitted in human cell lines (Kim et al., 2008b, 2010a). In fact, the benzoxathiol derivatives were synthesized for the development of anti-cancer drugs by targeting NF-κB signaling pathway, and BOT-4-one has anti-cancer and anti-inflammatory effects by inhibition of the pathways (not published yet). BOT-4-one decreased mRNA expression level of STAT3 in different types of human cancer cell lines, but the effect was stronger in Hodgkin's lymphoma cell line L540 compare to breast cancer cell line MDA-MB-468 and prostate cancer cell line DU145 (Figure 2A). In addition, the compound strongly inhibited mRNA expression and phosphorylation of STAT3 and STAT5 rather than those of STAT1 in L540 cells (Figure 2). However, mRNA expression levels of STAT1 and STAT5 in MDA-MB-468 and DU145 cells were not affected by BOT-4-one (Figure 2A). These results reveal that BOT-4-one has differential effect on the inhibition of STAT activation. As evidence for this hypothesis, BOT-4-one showed differential inhibition against JAK2 and JAK3 phosphorylation, and the effect was parallel compared to inhibition of STAT3 phosphorylation and STAT3 target protein SOCS3 expression. Non-receptor Src family kinases and ERK pathway can also regulate STAT3 phosphorylation (Garcia et al., 2001; Steelman et al., 2004). BOT-4-one strongly inhibited ERK1/2 phosphorylation in MDA-MB-468 and DU145 cells, but not in L540 cells. However, BOT-4-one showed weak effect on the activation of Src family kinases (Figure 3). We previously showed that JAK3 is important for STAT3-mediated signaling in L540 cells and JAK1 and JAK2 are important in MDA-MB-468 and DU145 cells (Kim et al., 2008b, 2010a, 2010b). Together, our results suggest that BOT-4-one has more selectivity for the regulation of JAK3/STAT3 signaling in L540 cells than that of JAK1/STAT3 and JAK2/STAT3 signaling in MDA-MB-468 and DU145 cells. This conclusion was further supported by reducing cell survival and inducing apoptosis through down-regulation of the expression of anti-apoptotic genes such as Bcl-2, Bcl-xL, Mcl-1, and survivin that are known to STAT3 downstream targets (Figures 4 and 5).
In summary, we identified a small molecule inhibitor of JAK/STAT signaling, especially JAK3/STAT3 signaling using Drosophila and human cancer cell lines. Inhibition of JAK3/STAT3 signaling by BOT-4-one decreased cancer cell survival, and induced apoptosis by down-regulation of anti-apoptotic gene expression in L540 cells. Therefore, BOT-4-one can be used as a lead compound to develop new group of anti-cancer drugs to target cancer cells harboring aberrant JAK3/STAT3 signaling.
Methods
Drosophila cell line, transfection and reporter assay
Maintenance of parental macrophage-like Drosophila Schneider (S2-NP) cells and reporter assay were conducted as previously described (Kim et al., 2008b, 2010a). Briefly, cells were cultured in Schneider's Drosophila medium containing 10% FBS and antibiotics (Invitrogen, Calsbad, CA) in an incubator at 25℃. S2-NP-STAT92E cells that stably express both the 10×STAT92E-firefly luciferase reporter gene and the PolIII-Renilla luciferase gene were also grown in the same medium supplemented with 500 µg/ml G418. For experiment STAT92E transcriptional activity, parental S2-NP cells were transiently transfected with Actin promoter-driven upd using Effectene Transfection Reagent (Qiagen, Valencia, CA) according to the manufacturer's protocol and the cells were co-cultured with S2-NP-STAT92E cells for 24 h in the presence of BOT-4-one at various concentrations. The reporter activity was quantified by measuring relative luciferase units (RLU) and the firefly luciferase activity was normalized to Renilla luciferase activity.
Human cancer cell lines
The Hodgkin's lymphoma cell line L540 and the Burkitt's lymphoma cell line DG-75 were purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany), and cultured in RPMI 1640 supplemented with 20% FBS and antibiotics. The breast cancer cell line MDA-MB-468 and the prostate cancer cell line DU145 were purchased from the American Type Culture Collection (Manassas, VA), and cultured in DMEM supplemented with 10% FBS and antibiotics. Cells were cultured in a 37℃ humidified incubator containing a mixture of 95% air and 5% CO2. DMEM, RPMI 1640, fetal bovine serum (FBS), and antibiotics (penicillin/streptomycin) were obtained from Invitrogen (Carlsbad, CA).
Reagents and antibodies
All reagents used in this experiment were obtained from Sigma-Aldrich (Saint Louis, MO), unless otherwise specified. Antibodies specific for phospho-STAT92E, phospho-STAT1 (Tyr701), phospho-STAT3 (Tyr705), phospho-STAT5 (Tyr694), phospho-JAK3 (Tyr980/981), phospho-JAK2 (Tyr1007/1008), JAK2, phospho-Src (Tyr416), Src, phospho-Lyn (Tyr507), phospho-ERK1/2 (Thr202/Tyr204), ERK1/2, PARP, caspase-3, Bcl-2, Bcl-xL, Mcl-1, survivin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling Technology (Danvers, MA) and used at a dilution of 1:1000-1:2500. Antibodies specific for STAT1, STAT3, STAT5, JAK3, Lyn and SOCS3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and used at a dilution of 1:500-1:2000. Mouse anti-HA antibody was purchased from Roche Applied Science (Indianapolis, IN). Secondary antibodies against from goat anti-rabbit and anti-mouse IgG horseradish peroxidase conjugate were purchased from Invitrogen (Calsbad, CA).
Immunoblot analysis
To analyze the effect of BOT-4-one on upd-induced STAT92E phosphorylation, S2-NP cells were transiently transfected with an expression plasmid for HA-tagged STAT92E and the cells were co-cultured with upd-producing cells in the presence of BOT-4-one (30 µM) for 24 h. Cell pellets were lysed with a lysis buffer (50 mM Tris-HCl, pH 7.4, 350 mM NaCl, 1% Triton X-100, 0.5% Nonidet P-40, 10% glycerol, 0.1% SDS, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 1 mM phenylmethylsulphonyl fluoride (PMSF) and phosphatase inhibitor cocktails) on ice. Proteins were separated by SDS-PAGE, transferred onto nitrocellulose membrane. Immunoblot analysis was performed with the phospho-STAT92E (1:1000 dilution) or HA antibody (1:1000 dilution). Human cancer cell pellets were lysed with a lysis buffer and membranes were prepared as described above. Immunoblot analysis was performed with appropriate primary antibody (1:1000 or 1:2500 dilution), horseradish peroxidase-conjugated secondary antibodies (1:5000 dilution), and an Enhanced Chemiluminescence Reagent System (iNtRON Biotechnology, Korea).
Cell viability, proliferation and FACS analysis
L540 cells (5×104 cells/ml) were treated with either vehicle (DMSO) alone or various concentrations of BOT-4-one in the presence or absence of IL-6 and incubated for the indicated time periods. Trypan blue exclusion assay was performed to count total and viable cells. Apoptosis assay was conducted using Terminal Transferase dUTP Nick End Labeling (TUNEL) assay system as previously described (Kim et al., 2008b, 2010a). Briefly, L540 cells (1.0×106 cells/ml) were treated with either vehicle (DMSO) alone or BOT-4-one (30 µM) for 48 h. Cells were harvested, stained using an APO-BRDU kit (Phoenix Flow Systems, Inc., San Diego, CA), and subsequently subjected to Elite ESP flow cytometry (Coulter Inc., Miami, FL).
RNA isolation and quantitative real-time PCR
Total RNA was isolated from human cancer cell lines treated with either vehicle (DMSO) alone or BOT-4-one for 24 or 48 h. For real-time PCR analysis, cDNA was synthesized from 1 µg of total RNA by reverse transcription using QuantiTect Rereverse Transcription Kit (Qiagen) and performed real time-PCR using the KAPA SYBR fast qPCR Kit (KAPA biosystems, Woburn, MA). Primers were purchased from Qiagen.
Statistical analysis
Data obtained from independent experiments are represented as means ± SD. Statistical analysis was performed using a two-tailed Student's t test. P values were considered to be statistically significant at *P < 0.001 or **P < 0.05.
Abbreviations
- BOT-4-one:
-
2-cyclohexylimino-6-methyl-6,7-dihydro-5H-benzo[1,3]oxathiol-4-one
- JAK:
-
janus kinase
- STAT3:
-
signal transducer and activator of transcription 3
- upd:
-
unpaired
References
Al Zaid Siddiquee K, Turkson J . STAT3 as a target for inducing apoptosis in solid and hematological tumors . Cell Res 2008 ; 18 : 254 - 267
Arbouzova NI, Zeidler MP . JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions . Development 2006 ; 133 : 2605 - 2616
Atallah E, Verstovsek S . Prospect of JAK2 inhibitor therapy in myeloproliferative neoplasms . Expert Rev Anticancer Ther 2009 ; 9 : 663 - 670
Bach EA, Vincent S, Zeidler MP, Perrimon N . A sensitized genetic screen to identify novel regulators and components of the Drosophila janus kinase/signal transducer and activator of transcription pathway . Genetics 2003 ; 165 : 1149 - 1166
Bercovich D, Ganmore I, Scott LM, Wainreb G, Birger Y, Elimelech A, Shochat C, Cazzaniga G, Biondi A, Basso G, Cario G, Schrappe M, Stanulla M, Strehl S, Haas OA, Mann G, Binder V, Borkhardt A, Kempski H, Trka J, Bielorei B, Avigad S, Stark B, Smith O, Dastugue N, Bourquin JP, Tal NB, Green AR, Izraeli S . Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down's syndrome . Lancet 2008 ; 372 : 1484 - 1492
Chang BY, Zhao F, He X, Ren H, Braselmann S, Taylor V, Wicks J, Payan DG, Grossbard EB, Pine PR, Bullard DC . JAK3 inhibition significantly attenuates psoriasiform skin inflammation in CD18 mutant PL/J mice . J Immunol 2009 ; 183 : 2183 - 2192
Chung EY, Kim BH, Lee IJ, Roh E, Oh SJ, Kwak JA, Lee YR, Ahn B, Nam SY, Han SB, Kim Y . The benzoxathiolone LYR-71 down-regulates interferon-gamma-inducible pro-inflammatory genes by uncoupling tyrosine phosphorylation of STAT-1 in macrophages . Br J Pharmacol 2009 ; 158 : 1971 - 1981
Darnell JE . STATs and gene regulation . Science 1997 ; 277 : 1630 - 1635
Decker P, Isenberg D, Muller S . Inhibition of caspase-3-mediated poly(ADP-ribose) polymerase (PARP) apoptotic cleavage by human PARP autoantibodies and effect on cells undergoing apoptosis . J Biol Chem 2000 ; 275 : 9043 - 9046
Fletcher S, Drewry JA, Shahani VM, Page BD, Gunning PT . Molecular disruption of oncogenic signal transducer and activator of transcription 3 (STAT3) protein . Biochem Cell Biol 2009 ; 87 : 825 - 833
Flex E, Petrangeli V, Stella L, Chiaretti S, Hornakova T, Knoops L, Ariola C, Fodale V, Clappier E, Paoloni F, Martinelli S, Fragale A, Sanchez M, Tavolaro S, Messina M, Cazzaniga G, Camera A, Pizzolo G, Tornesello A, Vignetti M, Battistini A, Cavé H, Gelb BD, Renauld JC, Biondi A, Constantinescu SN, Foà R, Tartaglia M . Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia . J Exp Med 2008 ; 205 : 751 - 758
Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Kraker A, Jove R . Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells . Oncogene 2001 ; 20 : 2499 - 2513
Goeth H, Wildfeuer A . Antimicrobial and cytostatic properties of 6-hydroxy-1,3-benzoxathiol-2-one (01 1) . Arzneimittelforschung 1969 ; 19 : 1298 - 1304
Haftchenary S, Avadisian M, Gunning PT . Inhibiting aberrant Stat3 function with molecular therapeutics: a progress report . Anticancer Drugs 2011 ; 22 : 115 - 127
Hazan-Halevy I, Harris D, Liu Z, Liu J, Li P, Chen X, Shanker S, Ferrajoli A, Keating MJ, Estrov Z . STAT3 is constitutively phosphorylated on serine 727 residues, binds DNA, and activates transcription in CLL cells . Blood 2010 ; 115 : 2852 - 2863
Herrmann A, Vogt M, Mönnigmann M, Clahsen T, Sommer U, Haan S, Poli V, Heinrich PC, Müller-Newen G . Nucleocytoplasmic shuttling of persistently activated STAT3 . J Cell Sci 2007 ; 120 : 3249 - 3261
Hou XS, Perrimon N . The JAK-STAT pathway in Drosophila . Trends Genet 1997 ; 13 : 105 - 110
Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, Kondo S, Priebe W, Kondo Y . A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo . Oncogene 2007 ; 26 : 2435 - 2444
James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W . A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera . Nature 2005 ; 434 : 1144 - 1148
Kim BH, Roh E, Lee HY, Lee IJ, Ahn B, Jung SH, Lee H, Han SB, Kim Y . Benzoxathiole derivative blocks lipopolysaccharide-induced nuclear factor-kappaB activation and nuclear factor kappaB-regulated gene transcription through inactivating inhibitory kappaB kinase beta . Mol Pharmacol 2008a ; 73 : 1309 - 1318
Kim BH, Yin CH, Guo Q, Bach EA, Lee H, Sandoval C, Jayabose S, Ulaczyk-Lesanko A, Hall DG, Baeg GH . A small-molecule compound identified through a cell-based screening inhibits JAK/STAT pathway signaling in human cancer cells . Mol Cancer Ther 2008b ; 7 : 2672 - 2680
Kim BH, Oh SR, Yin CH, Lee S, Kim EA, Kim MS, Sandoval C, Jayabose S, Bach EA, Lee HK, Baeg GH . MS-1020 is a novel small molecule that selectively inhibits JAK3 activity . Br J Haematol 2010a ; 148 : 132 - 143
Kim BH, Jee JG, Yin CH, Sandoval C, Jayabose S, Kitamura D, Bach EA, Baeg GH . NSC114792, a novel small molecule identified through structure-based computational database screening, selectively inhibits JAK3 . Mol Cancer 2010b ; 9 : 36 -
Kim JE, Kim HS, Shin YJ, Lee CS, Won C, Lee SA, Lee JW, Kim Y, Kang JS, Ye SK, Chung MH . LYR71, a derivative of trimeric resveratrol, inhibits tumorigenesis by blocking STAT3-mediated matrix metalloproteinase 9 expression . Exp Mol Med 2008c ; 40 : 514 - 522
Kim MH, Lee HY, Roh E, Kim BH, Chung EY, Lee YR, Lee IJ, Lee H, Lee CK, Han SB, Kim Y . Novel iminobenzoxathiolone compound inhibits nuclear factor-kappaB activation targeting inhibitory kappaB kinase beta and down-regulating interleukin-1beta expression in lipopolysaccharide-activated macrophages . Biochem Pharmacol 2008d ; 76 : 373 - 381
Klampfer L . Signal transducers and activators of transcription (STATs): Novel targets of chemopreventive and chemotherapeutic drugs . Curr Cancer Drug Targets 2006 ; 6 : 107 - 121
Lius V, Sennerfeldt P . Local treatment of acne with tioxolone . Lakartidningen 1979 ; 76 : 39 - 41
Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D, Carver-Moore K, DuBois RN, Clark R, Aguet M, Schreiber RD . Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway . Cell 1996 ; 84 : 431 - 442
Miyoshi K, Takaishi M, Nakajima K, Ikeda M, Kanda T, Tarutani M, Iiyama T, Asao N, DiGiovanni J, Sano S . Stat3 as a therapeutic target for the treatment of psoriasis: a clinical feasibility study with STA-21, a Stat3 inhibitor . J Invest Dermatol 2011 ; 131 : 108 - 117
Müller M, Briscoe J, Laxton C, Guschin D, Ziemiecki A, Silvennoinen O, Harpur AG, Barbieri G, Witthuhn BA, Schindler C, Pellegrini S, Wilks AF, Ihle JN, Stark GR, Kerr LM . The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction . Nature 1993 ; 366 : 129 - 135
Mullighan CG, Zhang J, Harvey RC, Collins-Underwood JR, Schulman BA, Phillips LA, Tasian SK, Loh ML, Su X, Liu W, Devidas M, Atlas SR, Chen IM, Clifford RJ, Gerhard DS, Carroll WL, Reaman GH, Smith M, Downing JR, Hunger SP, Willman CL . JAK mutations in high-risk childhood acute lymphoblastic leukemia . Proc Natl Acad Sci USA 2009 ; 106 : 9414 - 9418
Neubauer H, Cumano A, Müller M, Wu H, Huffstadt U, Pfeffer K . Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis . Cell 1998 ; 93 : 397 - 409
Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, Chang A, Kraker A, Jove R, Yu H . Roles of activated Src and Stat3 signaling in melanoma tumor cell growth . Oncogene 2002 ; 21 : 7001 - 7010
Oh ST, Simonds EF, Jones C, Hale MB, Goltsev Y, Gibbs KD, Merker JD, Zehnder JL, Nolan GP, Gotlib J . Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms . Blood 2010 ; 116 : 988 - 992
O'Shea JJ, Pesu M, Borie DC, Changelian PS . A new modality for immunosuppression: targeting the JAK/STAT pathway . Nat Rev Drug Discov 2004 ; 3 : 555 - 564
Park SY, Saijo K, Takahashi T, Osawa M, Arase H, Hirayama N, Miyake K, Nakauchi H, Shirasawa T, Saito T . Developmental defects of lymphoid cells in Jak3 kinase-deficient mice . Immunity 1995 ; 3 : 771 - 782
Schindler C, Shuai K, Prezioso VR, Darnell JE . Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor . Science 1992 ; 257 : 809 - 813
Schindler C, Plumlee C . Inteferons pen the JAK-STAT pathway . Semin Cell Dev Biol 2008 ; 19 : 311 - 318
Shuai K, Schindler C, Prezioso VR, Darnell JE . Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein . Science 1992 ; 258 : 1808 - 1812
Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA . JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis . Leukemia 2004 ; 18 : 189 - 218
Takeda K, Akira S . STAT family of transcription factors in cytokine-mediated biological responses . Cytokine Growth Factor Rev 2000 ; 11 : 199 - 207
Walters DK, Mercher T, Gu TL, O'Hare T, Tyner JW, Loriaux M, Goss VL, Lee KA, Eide CA, Wong MJ, Stoffregen EP, McGreevey L, Nardone J, Moore SA, Crispino J, Boggon TJ, Heinrich MC, Deininger MW, Polakiewicz RD, Gilliland DG, Druker BJ . Activating alleles of JAK3 in acute megakaryoblastic leukemia . Cancer Cell 2006 ; 10 : 65 - 75
Watling D, Guschin D, Müller M, Silvennoinen O, Witthuhn BA, Quelle FW, Rogers NC, Schindler C, Stark GR, Ihle JN, Kerr LM . Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway . Nature 1993 ; 366 : 166 - 170
Wildfeuer A . 6-hydroxy-1,3-benzoxathiol-2-one, an antipsoriatic with antibacterial and antimycotic properties . Arzneimittelforschung 1970 ; 20 : 824 - 831
Yu H, Pardoll D, Jove R . STATs in cancer inflammation and immunity: a leading role for STAT3 . Nat Rev Cancer 2009 ; 9 : 798 - 809
Acknowledgements
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1020160 and 0720540) and Mid-career Researcher Program (2010-0027827) through NRF grant funded by the MIST.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Experimental & Molecular Medicine website
Supplementary information
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kim, B., Min, Y., Choi, J. et al. Benzoxathiol derivative BOT-4-one suppresses L540 lymphoma cell survival and proliferation via inhibition of JAK3/STAT3 signaling. Exp Mol Med 43, 313–321 (2011). https://doi.org/10.3858/emm.2011.43.5.035
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2011.43.5.035
Keywords
This article is cited by
-
Involvement of inflammasomes in tumor microenvironment and tumor therapies
Journal of Hematology & Oncology (2023)
-
Mechanisms of PANoptosis and relevant small-molecule compounds for fighting diseases
Cell Death & Disease (2023)
-
Crosstalks between inflammasome and autophagy in cancer
Journal of Hematology & Oncology (2020)
-
Antileishmanial activity and immune modulatory effects of benzoxonium chloride and its entrapped forms in niosome on Leishmania tropica
Journal of Parasitic Diseases (2019)
-
OSMR gene effect on the pathogenesis of chronic autoimmune Urticaria via the JAK/STAT3 pathway
Molecular Medicine (2018)