Abstract
In this study, the essential oil from lotus flower extract, including petals and stamens, was assessed with regard to its effects on melanogenesis in human melanocytes. The lotus flower essential oil was shown to stimulate melanin synthesis and tyrosinase activity in a dose-dependent manner. The lotus flower essential oil induced the expression of tyrosinase, microphthalmia-associated transcription factor M (MITF-M), and tyrosinase-related proten-2 (TRP-2) proteins, but not tyrosinase mRNA. Moreover, it increased the phosphorylation of ERK and cAMP response element binding protein (CREB). In order to verify the effective components of the lotus flower oil, its lipid composition was assessed. It was found to be comprised of palmitic acid methyl ester (22.66%), linoleic acid methyl ester (11.16%), palmitoleic acid methyl ester (7.55%) and linolenic acid methyl ester (5.16%). Among these components, palmitic acid methyl ester clearly induced melanogenesis as the result of increased tyrosinase expression, thereby indicating that it may play a role in the regulation of melanin content. Thus, our results indicate that lotus flower oil may prove useful in the development of gray hair prevention agents or tanning reagents.
Similar content being viewed by others
Introduction
While we are looking for a new material to treat hypopimentation disorder, we choose lotus flower which is little consumed for food and study. Lotus (Nelumbo nuficera) is a perennial aquatic plant with yellow flowers. It is utilized as a dietary staple and also for a variety of medical purposes in Eastern Asia, particularly in China; the seeds of the lotus are utilized in the management of a variety of conditions, including tissue inflammation, poisoning, cancer, and leprosy. The plumule alleviates acute systemic inflammation in vivo (Lin et al., 2007) and the rhizomes have been shown to have antioxidant properties (Dongmei et al., 2007). The leaves are utilized to stanch bleeding in traditional Chinese medicine, and have also been shown to ameliorate hyperlipidemia in rodents (la Cour et al., 1995). The stamens of the lotus can be dried and made into a fragrant herbal Chinese tea, and evidence antioxidant effects in kidney homogenates (Jung et al., 2003). The petals of the lotus are utilized to impart a scent to tea leaves, primarily in Vietnam but also in other countries. Previously, palmitic acid as a predominant component of lotus plumule oil was analyzed (Bi et al., 2006). Palmitic acid is known to induce melanogenesis (Ando et al., 2004). However, there have thus far been no reports specifically addressing the effects of the lotus flower on melanogenesis.
Melanocytes perform a crucial role in photoprotection via the synthesis and transfer of melanin to keratinocytes, and this process is also relevant to the prevention of skin cancer. Melanin is derived from tyrosine with oxidation by a series of enzymes that includes tyrosinase, and is the major determinant of skin color. Constitutive skin color is genetically determined, but certain factors can alter skin color. Uneven skin color, caused either by hyperpigmentation or hypopigmentation, is a rather stressful condition for many afflicted individuals. In our search for a new drug for the treatment of hypopigmenation disorders in herbal medicine, we determined that the essential oil derived from an extract of the lotus flower, which included the petals and stamens, was effective with regard to melanogenesis in human melanocytes.
In the current study, we assessed the effects of lotus flower essential oil on proliferation and melanogenesis in human melanocytes, and assessed the essential oil components. The essential oil from lotus flower extract stimulates melanogenesis via an increase in microphthalmia-associated transcription factor M (MITF-M) and tyrosinase expression in human melanocytes. In this study, palmitic acid methyl ester was identified as a principal component of the lotus flower, and its effects on melanogenesis were verified.
Results
Effects of lotus flower essential oil on melanogenesis
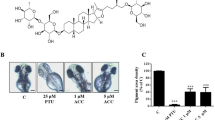
In order to determine the effects of the lotus flower on melanogenesis, melanocytes were treated for 5 days with four different concentrations of the extracts, 1.25, 2.5, 5, and 10 µg/ml. To compare the effect of BV on the melanocyte proliferation, we used forskolin, a cAMP elevating agent (Englaro et al., 1995) and the cAMP pathway is one of the most pivotal signaling pathways in melanogenesis. The melanin content was found to be increased by the lotus flower extracts in a dose-dependent manner (Figure 1A). At a lotus extract concentration of 10 µg/ml, the melanin contents of individual cells were approximately 2.1-fold as high as those in the untreated cells, which were themselves higher than those induced by forskolin treatment (Figure 1A). The number of cells was slightly increased at low dosage 1.25 and 2.5 µg/ml after treatment of lotus flower extracts. However, it showed a maximum effect of melanogenesis at 10 µg/ml of lotus extract (Figure 1A). Overall, lotus flower essential oil clearly enhances melanogenesis, but had no significant effects on proliferation.
Effects of lotus flower essential oil on melanogenesis in human melanocytes. Supplement-reduced melanocytes were untreated or treated with various concentrations of lotus flower oil or 10 µM forskolin (FK). After the melanocytes were incubated for 5 days, melanin content was determined and MTT assays were conducted (A). Supplement-reduced melanocytes were treated with 10 µg/ml of lotus flower essential oil for 0.5, 1, or 2 h, and for 1, 3, or 5 d. The expression of MITF-M, TRP-1, TRP-2, tyrosinase and GAPDH mRNA were assessed at the indicated times. The intensity of each band was quantitated by densitometry and normalized versus GAPDH. The error bars indicate S.E. (n = 4) (B). After treatment with lotus flower oil, tyrosinase and MITF-M protein expression were assessed at 1, 3, or 5 d. The intensity of each band was quantitated by densitometry and normalized versus β-actin. The data represent the means ± S.E. of three independent experiments (C).The error bars indicate S.E. (n = 5); *P < 0.01; **P < 0.001 (B).
Melanin is synthesized in melanosomes, which harbor the specific enzyme required for melanin production. Among them, the tyrosinase gene family has been determined to perform a crucial role in the regulation of melanogenesis (Pawelek et al., 1986), and this family includes tyrosinase, tyrosinase related protein (TRP-1) and dopachrome tautomerase (DCT, TRP-2). Tyrosinase is the specific enzyme that catalyzes the rate-limiting step for melanin biosynthesis. MITF-M is a melanocyte-specific transcription factor which controls pigmentation by regulating the expression of tyrosinase, TRP-1 and TRP-2 (Busca and Ballotti, 2000). Thus, the effects of lotus flower oil on melanogenesis were evaluated via determinations of MITF, tyrosinase, TRP-1, and TRP-2 expression, using western blot and RT-PCR techniques. After treatment with lotus flower extract (10 µg/ml), the levels of MITF-M mRNA expression was maximum at 1 h, and TRP-2 mRNA expression was also gradually increased until 2 h, indicating that MITF-M mRNA expression precedes TRP-2 expression (Figure 1B). However, the mRNA levels of tyrosinase and TRP-1 remained unaltered to 5 days (data not shown). Moreover, the protein levels of both MITF-M and tyrosinase were examined. MITF-M and tyrosinase protein expression gradually increased over 5 days of incubation (Figure 1C), indicating that MITF-M expression is correlated with mRNA induction and protein expression, whereas the expression of tyrosinase is not.
Composition of lotus flower essential oil and its effects on tyrosinase activity and expression
In order to ascertain which components are the principal effectors of melanogenesis, we analyzed the lotus flower essential oil with a GC-MS system. As shown in Table 1, there were nine chemicals to take more than 1% of total contents, with methyl hexadecanoate (palmitic acid methyl ester) and methyl cis,cis,9,12-octadecadienoate (linoleic acid methyl ester) as main components taking 22.66% and 11.16%, respectively. Linoleic acid, an unsaturated fatty acid, has been shown to reduce melanin contents in melanoma cells, whereas palmitic acid, a saturated fatty acid, increases it (Ando et al., 2004), thereby implying that although in the lotus flower oil extract, methyl groups stick to both fatty acids, the predomination of palmitic acid methyl ester may cause an increase in melanogenesis.
In an attempt to verify the effects of the primary components, palmitic acid methyl ester or linoleic acid methyl ester, on melanogenesis, they were administered to the melanocytes, and the results were compared with those of palmitic acid and linoleic acid, respectively. As has been previously reported (Nishizuka, 1992), linoleic acid and its methyl ester induced a slight reduction in the expression of tyrosinase in a dose-dependent manner (Figure 2A and B). Palmitic acid and its methyl ester induced a significant increase in tyrosinase expression, activity, and melanin content in a dose-dependent manner (Figure 2A, B and C). Moreover, the methyl ester of the palmitic acid evidenced less profound cytotoxic effects than were observed with palmitic acid (Figure 2C).
Effect of the main ingredients of lotus flower essential oil on tyrosinase activity and expression. Supplement-reduced melanocytes were stimulated with 10 µg/ml of lotus flower oil for 5 days in the presence of linolic acid (LA) 12.5 µM, linolic acid methyl ester (mLA) 12.5, 25, 50 µM, palmitic acid (PA) 12.5, 25, 50 µM or palmitic acid methyl ester (mPA) 12.5, 25, 50 µM. Tyrosinase expression was assessed. β actin was used as a control (A). The intensity of each band was quantitated by densitometry and normalized versus β-actin. The data represent the means ± S.E. of three independent experiments (B). Tyrosinase activity, melanin content and cell viability (MTT) were measured as described in the Methods section. The error bars indicate S.E. (n = 4) (C); *P < 0.01; **P < 0.001.
Signaling pathways activated by lotus flower essential oil
In the next step, we assessed the signaling pathways underlying the expression of MITF-M. MITF-M expression is up-regulated by PKA and simultaneously by the activation of cAMP response element binding protein (CREB) (Bertolotti et al., 1998). Moreover, CREB is also activated by ERKs (Englaro et al., 1995). Thus, in order to determine which signaling pathways are activated by the lotus flower in human melanocytes, we assessed the phosphorylation of ERKs and CREB using phosphospecific antibodies after the cells were treated with 10 µg/ml of essential oil from the lotus flower extracts. ERKs and CREB phosphorylation were increased at 30 min, and this persisted for 2 h after treatment with lotus flower essential oil (Figure 3A), thereby suggesting that lotus flower-induced MITF-M expression may occur via the activation of ERKs and CREB in melanocytes. Moreover, we examined whether ERKs and CREB pathway is activated by the methyl ester palmitic acid, a main component of lotus flower. It also phosphorylated ERKs but not CREB (Figure 3B, data not shown). In order to examine whether ERK activation is involved in the lotus flower or methyl ester palmitic acid-induced tyrosinase expression, PD98059, an inhibitor of MEK, which is an upstream kinase of ERK (Robinson and Cobb, 1997) was used. Lotus flower-induced ERK phosphorylation was effectively inhibited by pre-treatment of PD98059 (data not shown) but tyrosinase expression was not inhibited (Figure 3C).
Signaling pathways activated by lotus flower essential oil in human melanocytes. Supplement-reduced melanocytes were treated with 10 µg/ml of lotus flower essential oil (A) or 50 µM of palmitic acid methyl ester (mPA) (B) for 0.5, 1, or 2 h. The whole lysates were electrophoresed via SDS-PAGE and analyzed by immunoblotting with each antibody. The intensity of phosphorylation and total ERKs and CREB bands were quantitated by densitometry and the amounts of phosphorylated ERKs and CREB were normalized versus total ERKs and CREB. The data represent the means ± S.E. of four independent experiments. Supplement-reduced melanocytes were treated with 10 µg/ml of lotus flower essential oil (L) or 50 µM of palmitic acid methyl ester (mPA) in the absence or presence of 10 βM PD98059 (PD98059) for 2 days. The whole lysates were electrophoresed via SDS-PAGE and analyzed by immunoblotting with each antibody. Tyrosinase expression was normalized versus β-actin. The data represent the means ± S.E. of three independent experiments C: unstimulated melanocytes. *P < 0.01; **P < 0.001.
Discussion
In a recent study, we showed that bee venom increases melanogenesis in human melanocytes (Jeon et al., 2007). Moreover, it has been shown that extracts from some herbs, including glycyrrhizin (Jung et al., 2001), Kava rhizome extract (Matsuda et al., 2006) or quercetin (Nagata et al., 2004), increase melanogenesis in B16 melanoma cells, thereby indicating that natural resources should be extensively screened for the development of gray hair prevention agents or therapeutic drugs to induce repigmentation in the skin of vitiligo patients.
In this study, lotus flower extract was shown to enhance melanin synthesis in human melanocytes in a dose-dependent manner. Additionally, we have, for the first time, determined the active compounds in extracts of the lotus flower. The effects of lotus have been previously assessed with extracts of certain portions, using water, methanol, ethanol, chloroform, acetone, hexane, and/or dichloromethane (Borgi et al., 2007; Dongmei et al., 2007) or with dietary supplements (Lin et al., 2007), and several flavonoids or alkaloids have been identified as the active components of lotus plumules (Bi et al., 2006) or stamens (Jung et al., 2003). In addition to these components, the fatty acid composition of lotus plumule oil was analyzed; as saturated fatty acids, palmitic acid and behenic acid predominate, and as unsaturated fatty acids, linoleic acid and oleic acid predominate (Bi et al., 2006). Although the fatty acid compositions of the lotus plumule and the lotus flower are similar, the concentrations differ importantly; in the plumule, linoleic acid is the principal component (50.4%), followed by palmitic acid (18.0%) and oleic acid (13.5%), whereas in the flower, palmitic acid methyl ester predominates (22.66%), followed by linoleic acid methyl ester (11.16%), palmitoleic acid methyl ester (7.55%), and linolenic acid methyl ester (5.16%). It has been previously reported that linoleic acid reduces melanin content, whereas palmitic acid increases it via the regulation of tyrosinase ubiquitination without inducing mRNA level in melanoma cells (Ando et al., 1995, 2004) and palmitic acid conjugated melanotropic peptide increased tyrosinase activity compared with unconjugated (Hadley et al., 1991), which is concert with our result that the lotus flower including palmitic acid derivative as a major component, increased tyrosinase protein level without inducing mRNA level and ERK activation.
In the present study, we compared the melanogenic effects of palmitic acid and palmitic acid methyl ester in human melanocytes. Although palmitic acid methyl ester is less effective than palmitic acid with regard to tyrosinase expression and activity at the same dosage, cytotoxicity of each component was very different; the methyl ester of the palmitic acid showed little cytotoxic effects than were observed with palmitic acid at the same dosage, suggesting that the functional activity and toxicity of the derivative compound may differ from that of the original.
The tyrosinase gene family, tyrosinase, TRP-1, and TRP-2 share 70-80% nucleotide sequence homology, and 30-40% amino acid identity. Melanin synthesis is initiated by tyrosinase, which catalyzes the oxidation of tyrosine to DOPAchrome via a two-step reaction (Hearing and Tsukamoto, 1991). TRP-2 catalyzes the conversion of DOPAchrome to 5,6,dihydroxyindole-2-carboxylic acid, a precursor of brown melanins with low molecular weight and poor solubility (Tsukamoto et al., 1992; Solano et al., 1994). However, melanin may form even in the absence of TRP-2, as DOPAchrome can be non-enzymatically converted to an intermediate, 5,6-dihydroxyindole, which is capable of polymerizing into black, insoluble melanins. Therefore, TRP-2 has been theorized to function in the maintenance of cell viability in vivo via the control of the concentration of 5,6-dihydroxyindole, an extremely toxic intermediate, which in the absence of the enzyme would accumulate in the melanocytes (Pawelek et al., 1986). Cell-biological evidence indicates that TRP-2 is involved in cellular events associated with growth and morphology (Fang et al., 2001) and that its expression is dependent on cell-cell contact (Hornyak et al., 2000). In this study, the effects of lotus flower extract or palmitic acid methyl ester on the expression of tyrosinase mRNA remained unchanged, whereas lotus flower extract, but not palmitic acid, induced the expression of MITF-M and TRP-2 via the activation of CREB. Thus, the component of lotus flower extracts that induces CREB activation remains to be determined, as does the functional role of the increased MITF-M and TRP-2 in the melanocytes.
In summary, we have demonstrated that lotus flower essential oil may enhance melanogenesis via complex signaling pathways in normal human melanocytes, and that palmitic acid methyl ester, as its principal component, may perform a role in the regulation of melanin content. Thus, our results indicate that lotus flower extracts may potentially be used in the development of gray hair prevention agents or tanning reagents.
Methods
Materials
Flowers of Nelumbo nuficera, including the petals and stamens, were purchased from a local market in Ilsan, Gyeonggi-do. Methyl cis,cis-9,12-octadecadienoic acid (linoleic acid methyl ester), octadecadienoate (linoleic acid), hexadecanoic acid (palmitic acid), and methyl hexadecanoic acid (palmitic acid methyl ester) were acquired from the Sigma Co. (St. Louis, MO). Forskolin was purchased from Calbiochem (Fontenay sous Bois, France).
Preparation of lotus flower oil extract
The dried and pulverized lotus flowers (87 g) were extracted using 2 L of n-hexane for 48 h at room temperature then filtered. The filtrates were evaporated at 50℃ in order to remove the hexane. The hexane was further eliminated in vacuo for 30 min at room temperature, yielding a clear yellowish essential oil (1.2 g). For treatment in melanocytes, the essential lotus flower oil was dissolved in DMSO (dimethylsulfoxide) at a concentration of 10 g/L, and was then filtered using a 0.2 µm syringe filter.
GC/MS analysis
For the qualitative and quantitative analysis of the essential oil components, gas chromatography-mass spectrometry (GC/MS) was conducted on a Hewlett Packard 5890 series II instrument connected to an Automass 50 (JEOL). The operating conditions were as follows: column--fused silica capillary column, TC-wax (Hewlett Packard), 60 m × 0.25 mm, film thickness = 0.25 µm; column temperature: 40-300℃ increasing at 5℃/min to 150℃, then at 15℃/min to 300℃, ending at 300℃ for 5 min; injector: 180℃; carrier gas: nitrogen at a flow rate of 30 cm/s; column head pressure: 180 kPa; injection volume: 0.5 ml; ionization energy: 70 eV; ion source temperature: 200℃. The chemical components were identified via comparison of their retention times and mass spectra with those in the MS data library (NBS library). The relative quantity of each component was determined by calculating the peak area of the TIC chromatogram.
Normal human epidermal melanocyte culture
Skin specimens obtained from repeated cesarean sections and circumcisions were employed for cultures. The cells were suspended in Medium 254 (#M-254-500; Cascade Biologics, Portland, OR) supplemented with bovine pituitary extract, FBS, bovine insulin, hydrocortisone, basic fibroblast growth factor (bFGF), and bovine transferrin (#S-001-5; Cascade Biologics, Portland, OR), heparin, and PMA (#S-002-5; Cascade Biologics, Portland, OR).
MTT assay
Melanocyte proliferation was assessed with an MTT assay kit (R&D Systems, Minneapolis, MN). Each absorbance was measured at a wavelength of 570 nm. The MTT assay was conducted with melanocytes treated with lotus flower essential oil, each of its components, forskolin (10 µm), or nothing.
Measurement of melanin content
The melanin content of the cultured melanocytes was evaluated in accordance with the method described by Oka et al. (1996). In brief, the cell pellets were solubilized in 1 M NaOH (80℃) boiled for 2 h. The color was analyzed at a wavelength of 475 nm. In order to determine the actual melanin formation from the same number of cells, the total melanin content of each pellet was divided by the number of melanocytes.
Assay of tyrosinase activity
Tyrosinase activity was assayed in terms of 3,4-dihydroxyphenylalanine (DOPA) oxidase activity, using a modified version of the described method (Takahashi and Parsons, 1992). The cells were washed twice in PBS and lysed with phosphate buffer containing 1% Triton X-100. The lysates were clarified via 10 min of centrifugation at 10,000 × g. After quantifying the protein levels via Bradford assays and adjusting the concentrations with lysis buffer, 90 µl of each lysate was placed in a well of a 96-well plate, and 10 µl of L-DOPA was added. After incubation at 37℃, absorbance was measured every 10 min for at least 1 h at 475 nm with an ELISA reader.
RT-PCR
Total RNA from melanocytes cultured under each condition was isolated with QuickGene RNA cultured cell HC kits (Life Science, Minato-ku, Tokyo, Japan). cDNA was synthesized from the total RNA using the First Strand cDNA Synthesis Kit for RT-PCR (AMV; Boehringer Mannheim, Germany). Human GAPDH was utilized as an internal standard. The primer sequences of MITF were as follows: forward 5'-ATGCTGGAAATGCTAGAATATAAT-3'; and reverse 5'-ATCATCCATCTGCATACAG-3'; TRP-1 (forward 5'-TGGCAAAGCGCACAACTCACCC-3' and reverse 5'-AGTGCAACCAGTAACAAAGCGCC-3') TRP-2 (5'-GCACACATGTAACCTCTGTG-3' and reverse 5'-TCATATAAGCAGGCTTGGCC-3') tyrosinase (forward 5'-TTGGCATAGACTCTTCTTGTTGCGG-3' and reverse 5'-CAAGGAGCCATGACCAGATCCG-3') and GAPDH (forward 5'-TCCACTGGCGTCTTCACC-3'; and reverse 5'-GGCAGAGATGATGACCCTTTT-3'). PCR amplification was conducted in a 40 µl reaction volume, consisting of 10 × reaction buffer, 2.5 mM MgCl2, 250 µM dNTPs, 100 ng of each PCR primer, 0.5 U of Taq DNA polymerase, and 20-50 ng of DNA, using a DNA Thermal Cycler 9600 (Applied Biosystems, Foster City, CA). The DNA fragments generated by PCR were separated via electrophoresis on 2% agarose gel. The bands were analyzed via densitometry.
Western blot analysis
The cultured melanocytes treated with the lotus flower essential oil or forskolin with or without inhibitor were homogenized in ice-cold homogenization buffer containing 50 mM Tris-base (pH 7.4), 150 mM NaCl, 10 mM EDTA, 0.1% Tween-20, and protease inhibitors. Equal amounts of extracted proteins (30 µg) were resolved via 10% SDS-PAGE and transferred to nitrocellulose membranes. After incubation in a blocking solution of 5% nonfat dry milk in Tris-buffered saline (TBS) containing 10 mM Tris (pH 7.6), 150 mM NaCl, and 0.1% Tween-20, the membranes were incubated overnight at 4℃ with anti-phospho-ERK, ERK, CREB, phospho-CREB (rabbit polyclonal; Cell Signaling Technology, Beverly, MA), MITF (mouse monoclonal; Abcam, Cambridge, UK) or tyrosinase (rabbit polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA) antibodies, and diluted to 1:1000 in blocking solution. The membranes were then further incubated with anti-rabbit or anti-mouse HRP-antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and treated with an enhanced chemiluminescence solution (Pierce kit; Rockford, IL). The signals were then captured on an Image Reader (LAS-3000; Fuji Photo Film, Tokyo, Japan). In order to monitor the quantity of protein loaded into each lane, the membranes were reprobed with mouse monoclonal anti-actin antibody (Sigma, St. Louis, MO) and processed as described above. The protein bands were analyzed via densitometry.
Statistical analysis
Statistical significance was tested with Student t-test. All results are presented as the mean ± S.E. of the combined data from replicate experiments.
Abbreviations
- CREB:
-
cAMP response element binding protein
- MITF-M:
-
microphthalmia-associated transcription factor M
- TRP-1:
-
tyrosinase-related proten-1
- TRP-2:
-
tyrosinase-related proten-2
References
Ando H, Itoh A, Mishima Y, Ichihashi M . Correlation between the number of melanosomes, tyrosinase mRNA levels, and tyrosinase activity in cultured murine melanoma cells in response to various melanogenesis regulatory agents . J Cell Physiol 1995 ; 163 : 608 - 614
Ando H, Watabe H, Valencia JC, Yasumoto K, Furumura M, Funasaka Y, Oka M, Ichihashi M, Hearing VJ . Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: a new aspect of ubiquitin-proteasome function . J Biol Chem 2004 ; 279 : 15427 - 15433
Bertolotti A, Melot T, Acker J, Vigneron M, Delattre O, Tora L . EWS, but not EWS-FLI-1, is associated with both TFIID and RNA polymerase II: interactions between two members of the TET family, EWS and hTAFII68, and subunits of TFIID and RNA polymerase II complexes . Mol Cell Biol 1998 ; 18 : 1489 - 1497
Bi Y, Yang G, Li H, Zhang G, Guo Z . Characterization of the chemical composition of lotus plumule oil . J Agric Food Chem 2006 ; 54 : 7672 - 7677
Borgi W, Bouraoui A, Chouchane N . Antiulcerogenic activity of Zizyphus lotus (L.) extracts . J Ethnopharmacol 2007 ; 112 : 228 - 231
Busca R, Ballotti R . Cyclic AMP a key messenger in the regulation of skin pigmentation . Pigment Cell Res 2000 ; 13 : 60 - 69
Dongmei Y, Qiushuang W, Leqin K, Jianmei j, Tiejin Y . Vitamin A status of the minority ethnic group of Karen hill tribe children aged 1-6 years in Northern Thailand . Asia Pac J Clin Nutr 2007 ; 16 : 158 - 162
Englaro W, Rezzonico R, Durand-Clément M, Lallemand D, Ortonne JP, Ballotti R . Mitogen-activated protein kinase pathway and AP-1 are activated during cAMP-induced melanogenesis in B-16 melanoma cells . J Biol Chem 1995 ; 270 : 24315 - 24320
Fang D, Kute T, Setaluri V . Regulation of tyrosinase-related protein-2 (TYRP2) in human melanocytes: relationship to growth and morphology . Pigment Cell Res 2001 ; 14 : 132 - 139
Hadley ME, al-Obeidi F, Hruby VJ, Weinrach JC, Freedberg D, Jiang JW, Stover RS . Biological activities of melanotropic peptide fatty acid conjugates . Pigment Cell Res 1991 ; 4 : 180 - 185
Hearing VJ, Tsukamoto K . Enzymatic control of pigmentation in mammals . FASEB J 1991 ; 5 : 2902 - 2909
Hornyak TJ, Hayes DJ, Ziff EB . Cell-density-dependent regulation of expression and glycosylation of dopachrome tautomerase/tyrosinase-related protein-2 . J Invest Dermatol 2000 ; 115 : 106 - 112
Jeon S, Kim NH, Koo BS, Lee HJ, Lee AY . Bee venom stimulates human melanocyte proliferation, melanogenesis, dendricity and migration . Exp Mol Med 2007 ; 39 : 603 - 613
Jung GD, Yang JY, Song ES, Par JW . Stimulation of melanogenesis by glycyrrhizin in B16 melanoma cells . Exp Mol Med 2001 ; 33 : 131 - 135
Jung HA, Kim JE, Chung HY, Choi JS . Antioxidant principles of Nelumbo nucifera stamens . Arch Pharm Res 2003 ; 26 : 279 - 285
la Cour B, Mølgaard P, Yi Z . Traditional Chinese medicine in treatment of hyperlipidaemia . J Ethnopharmacol 1995 ; 46 : 125 - 129
Lin JY, Lai YS, Liu CJ, Wu AR . Effects of lotus plumule supplementation before and following systemic administration of lipopolysaccharide on the splenocyte responses of BALB/c mice . Food Chem Tox 2007 ; 45 : 486 - 493
Matsuda H, Hirata N, Kawaguchi Y, Naruto S, Takata T, Oyama M, Iinuma M, Kubo M . Melanogenesis stimulation in murine B16 melanoma cells by Kava (Piper methysticum) rhizome extract and kavalactones . Biol Pharm Bull 2006 ; 29 : 834 - 837
Nagata H, Takekoshi S, Takeyama R, Homma T, Yoshiyuki Osamura R . Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and in normal human melanocytes . Pigment Cell Res 2004 ; 17 : 66 - 73
Nishizuka Y . Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C . Science 1992 ; 258 : 607 - 614
Oka M, Ichihashi M, Chakraborty AK . Enhanced expression of protein kinase C subspecies in melanogenic compartments in B16 melanoma cells by UVB or MSH . J Invest Dermatol 1996 ; 106 : 377 - 378
Pawelek JM, Murray M . Increase in melanin formation and promotion of cytotoxicity in cultured melanoma cells caused by phosphorylated isomers of L-dopa . Cancer Res 1986 ; 46 : 493 - 497
Robinson MJ, Cobb MH . Mitogen-activated protein kinase pathways . Curr Opin Cell Biol 1997 ; 9 : 180 - 186
Solano F, Martinez-Liarte JH, Jimenez-Cervantes C, Garcia-Borron JC, Lozano JA . Dopachrome tautomerase is a zinc-containing enzyme . Biochem Biophys Res Commun 1994 ; 204 : 1243 - 1250
Takahashi H, Parsons PG . Rapid and reversible inhibition of tyrosinase activity by glucosidase inhibitors in human melanoma cells . J Invest Dermatol 1992 ; 98 : 481 - 487
Tsukamoto K, Jackson IJ, Urabe K, Montague PM, Hearing VJ . A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase . EMBO J 1992 ; 11 : 519 - 526
Acknowledgements
This study was supported by a grant of the Korea Healthcare technology R&D Project (A080980), Ministry of Health Welfare and Family Affairs, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Jeon, S., Kim, NH., Koo, BS. et al. Lotus (Nelumbo nuficera) flower essential oil increased melanogenesis in normal human melanocytes. Exp Mol Med 41, 517–524 (2009). https://doi.org/10.3858/emm.2009.41.7.057
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2009.41.7.057
Keywords
This article is cited by
-
Chemical Composition and Tyrosinase Inhibitory Activities of Fatty Acids Obtained from Heterotrophic Microalgae, S. limacinum and C. cohnii
Applied Biochemistry and Biotechnology (2023)
-
Red alert about lipid's role in skin cancer
Nature (2017)