Abstract
Angiotensin II (Ang II) stimulates migration of vascular smooth muscle cell (VSMC) in addition to its contribution to contraction and hypertrophy. It is well established that Rho GTPases regulate cellular contractility and migration by reorganizing the actin cytoskeleton. Ang II activates Rac1 GTPase, but its upstream guanine nucleotide exchange factor (GEF) remains elusive. Here, we show that Ang II-induced VSMC migration occurs in a βPIX GEF-dependent manner. βPIX-specific siRNA treatment significantly inhibited Ang II-induced VSMC migration. Ang II activated the catalytic activity of βPIX towards Rac1 in dose- and time-dependent manners. Activity reached a peak at 10 min and declined close to a basal level by 30 min following stimulation. Pharmacological inhibition with specific kinase inhibitors revealed the participation of protein kinase C, Src family kinase, and phosphatidylinositol 3-kinase (PI3-K) upstream of βPIX. Both p21-activated kinase and reactive oxygen species played key roles in cytoskeletal reorganization downstream of βPIX-Rac1. Taken together, our results suggest that βPIX is involved in Ang II-induced VSMC migration.
Similar content being viewed by others
Introduction
Angiotensin II (Ang II) is a critical regulator of vascular remodeling associated with various vascular pathophysiologies; thus, its signaling has become the subject of great interest (Zheng et al., 2006; Higuchi et al., 2007; Mehta and Griendling, 2007). Most of the Ang II stimulus is introduced into target cells including vascular smooth muscle cells (VSMCs) via the Ang II type I (AT1) receptor. Downstream of the AT1 receptor, Ang II activates multiple signaling pathways, which leads to contraction, hypertrophy and migration of VSMCs (Higuchi et al., 2007; Mehta and Griendling, 2007). Although signaling pathways for contraction and hypertrophy have been intensively studied, relatively little is known concerning migration.
Rho GTPases regulate a wide range of cellular processes including cell proliferation, migration and morphological changes. Initial studies revealed their critical roles in actin remodeling: RhoA, Rac1 and Cdc42 form stress fibers, lamellipodia and filopodia, respectively (Hall, 1998). Furthermore, the RhoA/ROCK pathway contributes to cellular contractility, which also mediates Ang II-induced contraction of VSMCs (Seko et al., 2003). Recently, Ohtsu et al., (2005) reported that Ang II-induced migration of VSMCs utilizes crosstalk of RhoA-ROCK and JNK. Upstream of this pathway, RhoA-activating guanine nucleotide exchange factors (GEFs) such as PDZRhoGEF and Vav are considered to act in a G12/13-dependent manner (Wakino et al., 2004; Ohtsu et al., 2005). It is rather surprising that the RhoA-ROCK pathway mediates both VSMC contraction and migration, which seemingly counteract each other. Rac1, another Rho GTPase, is also activated by Ang II in VSMCs and utilizes the p21-activated kinase (PAK)-JNK pathway (Schmitz et al., 2001) and NAD(P)H oxidase (Seshiah et al., 2002). This pathway has been shown to promote cell growth, but its role for migration remains poorly understood. Moreover, a GEF(s) upstream of Rac1 activation in Ang II-induced VSMC migration has not yet been addressed.
βPIX is a Rac1/Cdc42-specific GEF that forms a tight complex with PAK (Manser et al., 1998). The PAK family is divided into two subgroups depending on the distinct structural and functional properties. PAK1-3 and PAK4-6 belong to group I and II, respectively. When activated by growth factors, PAK2 can readily phosphorylate βPIX (Shin et al., 2002), which in turn stimulates its GEF activity towards Rac1/Cdc42 (Shin et al., 2004, 2006). Ang II activates PAK1 in VSMCs suggesting the potential involvement of PAK/βPIX/Rac1 in Ang II-induced VSMC migration. In response to growth factor stimulation, Rac1 is activated in a βPIX-dependent manner (Park et al., 2004; Shin et al., 2004). Activated Rac1 in turn stimulates NAD(P)H oxidase, resulting in generation of reactive oxygen species (ROS), such as superoxide anions and hydrogen peroxide (Park et al., 2004). ROS mediates many cellular functions including hypertrophy, migration and contraction of VSMCs through activation of multiple downstream signaling molecules such as tyrosine kinases/phosphatases and MAPKs (Lyle and Griendling, 2006). The present study was undertaken to investigate a potential role for βPIX in the Ang II signaling. Here, we demonstrate that βPIX contributes to Ang II-induced VSMC migration via two distinct signaling pathways, Rac1-PAK1 and Rac1-NAD(P)H oxidase.
Results
Rac1 is involved in Ang II-induced migration of VSMCs
Rac1 controls actin dynamics for membrane protrusion and ruffling, which is required for cell migration (Ridley, 2006). Appropriately, we tested whether Rac1 could mediate Ang II-induced migration in VSMCs. VSMCs were pretreated with the specific Rac1 inhibitor NSC23766 (Gao et al., 2004) and were stimulated with Ang II. Ang II-induced migration was inhibited by NSC23766 in a dose-dependent manner (Figure 1A). In the absence of NSC23766, relative VSMC migration was 2.72 times that of the control. However, an inhibitor concentration of 50 µM produced a decline of relative migration to 1.25, representing a 25% increase over the control. Rac1 is targeted to the plasma membrane and focal adhesion sites (ten Klooster et al., 2006; Chang et al., 2007). Appropriately, we investigated whether localization of Rac1 was altered in response to Ang II stimulation. In serum-starved quiescent cells, Rac1 was mainly localized at the perinuclear area (Figure 1B). At 10 min following Ang II stimulation, Rac1 appeared at the plasma membrane. In parallel, targeting lamellipodia and membrane ruffles were prominent. Up to 30 min these actin structures were observed co-localized with Rac1. These results suggest that Rac1 is activated and targeted to the membrane, where it mediates migration of VSMCs in response to Ang II stimulation.
Rac1 mediates Ang II-induced migration of VSMCs. (A) Inhibitory effect of NSC23766 on VSMC migration. Cells were grown to confluence and scratch wounds were made. At 12 h following treatment with none (control), Ang II alone (1 µM), or Ang II + NSC23766 (NSC) of the indicated concentrations, the wound edges were photographed (upper) and relative migration was analyzed using MetaMorph software (bottom). Migration rate of the unstimulated cells was set to 1. Data are expressed as mean ± SD. (B) Targeting of Rac1 to the plasma membrane. Cells were stimulated with 100 nM Ang II for the indicated times and stained with anti-Rac1 antibody (green) and TRITC-conjugated phalloidin (red) for actin. Representative images from three independent experiments are shown here. Arrows (white) indicate the plasma membrane where Rac1 is enriched. Scale bar, 10 µM.
βPIX functions upstream of Rac1 in Ang II-induced migration of VSMCs
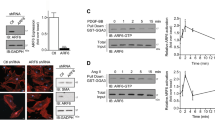
It is unknown which GEF(s) activate Rac1 in the Ang II signaling in VSMCs. βPIX is ubiquitously expressed and regulates migration in diverse types of cells. βPIX isoforms may play important roles in migration of neuronal cells during embryonic development (Kim et al., 2000). Moreover, βPIX controls motility of fibroblasts (Cau and Hall, 2005; Za et al., 2006), epithelial cells (Nola et al., 2008) and T cells (Volinsky et al., 2006). We determined whether βPIX contributes to Ang II-induced VSMC migration. Cells were incubated with scrambled (control) or βPIX-specific siRNAs for 2 days and their Ang II-induced migration was measured 12 h following stimulation. As illustrated in Figure 2A (right), treatment with βPIX-specific siRNA resulted in successful silencing of βPIX. Scrambled siRNA-treated cells showed a similar migration profile as that from non-treated cells (Figure 2A, left and center). In contrast, the relative migration rate of βPIX siRNA-treated cells showed only a 1.7-fold increase compared to unstimulated cells (Figure 2A, right). These results strongly support the suggestion that βPIX might be an unidentified component in the signaling pathway of Ang II-induced migration.
βPIX mediates Ang II-induced migration of VSMCs. (A) Inhibitory effect of βPIX depletion on VSMC migration. Cells were transfected with βPIX-specific siRNA (100 nM) or scrambled siRNA. After 48 h, the wound migration assay was conducted. Representative images are shown (left) and relative migration data are expressed as mean ± SD (center). Expression of βPIX was confirmed by Western blotting (right). (B) βPIX-dependent Rac1 activation and targeting by Ang II. Cells were stimulated with 100 nM Ang II for the indicated times (left) or with increasing concentrations for 10 min (center). Cell lysates were immunoprecipitated with anti-βPIX antibody followed by incubation with 2 µg purified soluble GST-PBD. Bound GST-PBD representing active Rac1 in the immunoprecipitates was detected by Western blotting with anti-GST antibody (top). Immunopreciptated βPIX and bound Rac1 were confirmed by immunoblotting with anti-βPIX (bottom) and Rac1 (middle) antibodies, respectively. To analyze βPIX-dependent Rac1 targeting by Ang II, cells were transfected with the indicated siRNAs for 48 h (right). Membrane fractions from siRNA-treated cells were immunoblotted with anti-Rac1 (top) or βPIX (bottom) antibody.
To determine whether Ang II could stimulate Rac1 activation in a βPIX-dependent manner, we measured Rac1 activity with a modification of conventional PBD-pulldown assay (Shin et al., 2004). This modified protocol takes advantage of the tight association between βPIX and Rac1. Following Ang II stimulation, βPIX-Rac1 complex can be precipitated from lysates using anti-βPIX antibody, and active Rac1-GTP in this complex is subsequently detected using soluble GST-PBD. Hence, Rac1 activation can be monitored indirectly by measuring bound soluble GST-PBD using immunoblotting with anti-GST antibody. Rac1 activation reached a peak 10 min following Ang II stimulation and gradually declined thereafter (Figure 2B, left). Ang II rapidly activates Rac1 within a minute (Schmitz et al., 2001) and is sustained up to 30 min (Seshiah et al., 2002). However, presently the early activation peak was not observed in βPIX-dependent Rac1 activation. These results suggest involvement of other GEF(s) in the early peak. βPIX appeared to be responsible for Rac1 activation at a later time. βPIX-dependent Rac1 activation increased up to 1 µM in a dose-dependent manner (Figure 2B, middle). We further determined whether Rac1 targeting to the plasma membrane occurred in a βPIX-dependent manner. Cells were treated with siRNA and then stimulated with Ang II. In untreated and scrambled siRNA-treated cells, Rac1 was greater in Ang II-stimulated membranes (Figure 2B, right). In contrast, this response was not observed in βPIX siRNA-treated cells.
PKC, Src family kinase and phosphatidylinositol 3-kinase function upstream of βPIX in Ang II-stimulated Rac1 activation
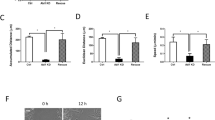
Ang II activates Rac1, whose upstream kinases include PKC, Src family kinase (Src) and phosphatidylinositol 3-kinase (PI3-K) (Seshiah et al., 2002; Lyle and Griendling, 2006). To determine whether these kinases could activate Rac1 via βPIX, VSMCs were pretreated with specific kinase inhibitors (GF109203X for PKC, PP2 for Src and LY294002 for PI3-K) prior to stimulation with Ang II. As described above in Figure 2B, βPIX-dependent Rac1 activation occurred by 10 min following exposure to Ang II (Figure 3A). Treatment with these inhibitors abolished the βPIX-dependent Rac1 activation. These results strongly suggest that PKC, Src and PI3-K regulate the activation of βPIX, although it is not clear how they sequentially act on βPIX. PAK1 is a well-known downstream effector of Rac1; hence, we checked whether these kinases also function upstream of PAK1. Phosphorylation of Thr423 of PAK1 is essential for its activation (Zenke et al., 1999). We monitored PAK1 activation following inhibitor treatment using an antibody that specifically recognizes this phospho-PAK1. As shown in Figure 3B, phospho-PAK1 almost disappeared upon the inhibition of Src but not upon inhibition of PKC and PI3-K. Ang II-stimulated Rac1 also mediates ROS generation through activation of NAD(P)H oxidase. We determined whether ROS generation could be affected by the tested kinase inhibitors. VSMCs were exposed to Ang II in the presence or absence of the inhibitors. All three inhibitors considerably reduced Ang II-stimulated ROS generation (Figure 3C). Taken together, these results suggest that, although PKC, Src and PI3-K contribute to βPIX-dependent Rac1 activation, they differentially regulate PAK1- or NAD(P)H oxidase-mediated downstream events.
Upstream activators of the βPIX-Rac1 pathway in the Ang II signaling. (A) Effect of kinase inhibitors on βPIX-dependent Rac1 activation by Ang II. VSMCs were treated with 10 µM PP2, 10 µM GF109236X (GFx) or 50 µM LY294002 (LY) for 1 h prior to stimulation with 100 nM Ang II for 10 min. Cells were then processed as described in Figure 2B. (B) Effect of kinase inhibitors on Ang II-stimulated PAK1 activation. Cells were treated as described above and lysates were immunoblotted with anti-phospho PAK1 (T423-P) (top) and total PAK1 antibodies (bottom). (C) Effect of kinase inhibitors on Ang II-stimulated ROS production. Cells were treated as described above except addition of 10 µM N-acetyl cysteine (NAC) as a positive control. To measure ROS production cells were incubated with 1 µM DCF-DA for 10 min in the dark. ROS-mediated oxidation of DCF dye was determined by monitoring changes in the intensity of fluorescence by inverted fluorescence microscopy. Representative images are shown (left). Scale bar, 20 µM. Digitalized data of the fluorescence from 10 randomly selected fields were analyzed by MetaMorph software (right). Basal levels of ROS production were set to 1 and relative values were presented as mean ± SD.
PAK1 and NAD(P)H oxidase function downstream of βPIX-Rac1 in the Ang II signaling pathway
Although the above results were consistent with the suggestion that PKC, SRC and PI3-K act as upstream regulators for PAK1 activation and ROS generation, it was not clear whether βPIX mediates this process. Using siRNA technology, we tested the involvement of βPIX. VSMCs were treated with scrambled or βPIX siRNA for 2 days and then stimulated with Ang II. PAK1 activation and ROS generation were determined by monitoring the status of phospho-PAK1 and alteration in fluorescence intensity of DCF, respectively. Treatment with scrambled siRNA had little effect on Ang II-stimulated PAK activation. In contrast, depletion of βPIX significantly inhibited PAK1 activation (Figure 4A). Scrambled siRNA-treated cells displayed a 3-fold increase in relative intensity of DCF fluorescence following exposure to Ang II as compared to unstimulated cells (Figure 4B). However, in βPIX-depleted cells DCF fluorescence was increased only 1.68-fold in response to Ang II stimulation. Collectively, these results indicate that βPIX mediates PAK1 activation and ROS generation.
PAK1 and ROS production downstream of βPIX in the Ang II signaling. (A) βPIX-dependent PAK1 activation by Ang II. VSMCs were transfected with scrambled siRNA or βPIX-specific siRNA (100 nM). After 48 h, cells were treated with 100 nM Ang II for 10 min. Cell lysates were immunoblotted with anti-phospho PAK1 (T423-P), total PAK1, βPIX. For a loading control the same blot was reprobed with anti-GAPDH antibody. (B) βPIX-dependent ROS production by Ang II. VSMCs were treated with siRNA as described above and ROS production was measured as described in Figure 3C (left). siRNA effects were confirmed by monitoring the expression levels of βPIX with immunoblotting (right).
Discussion
In the present study we have demonstrated that βPIX exchange factor plays a key role in Ang II-stimulated Rac1 activation, which induces cytoskeletal reorganization required for efficient migration of VSMCs. The βPIX-Rac1 pathway utilized PAK1 and NAD(P)H oxidase as downstream effectors (Figure 4). Proper spatio-temporal localization of Rac1 is critical for activation of these downstream effectors. In adherent cells upon attachment to matrix Rac1 is targeted to cholesterol-enriched membrane microdomains/lipid rafts where Rac1 activates PAK1 (del Pozo et al., 2004). Even though Rac1 is activated in cell suspensions, PAK1 activation is barely detected (del Pozo et al., 2000). βPIX targets Rac1 to membrane ruffles and focal adhesion sites (ten Klooster et al., 2006). Consistent with this, following Ang II stimulation we observed Rac1 recruitment to the plasma membrane (Figure 1B), and its targeting and activation was βPIX-dependent (Figure 2B). In focal adhesion kinase (FAK) null cells, targeting of Rac1 to focal adhesion sites seldom occurs suggesting the involvement of FAK in Rac1 targeting (Chang et al., 2007). Moreover, FAK mediates this process through tyrosine phosphorylation of βPIX. NAD(P)H oxidase is also activated in caveolae/caveolin-1-enriched lipid rafts (Zuo et al., 2004). Thus, one can expect that Ang II-stimulated Rac1 and NAD(P)H oxidase activation critically requires caveolin-1 (Zuo et al., 2005). Interestingly, in quiescent VSMCs AT1 receptor is detected in cholesterol-independent microdomains, but its relocalization to caveolae occurs upon activation by Ang II (Wyse et al., 2003). The AT1 receptor in turn transactivates EGF receptor, which leads to activation of multiple downstream effectors such as Ras-ERK and PI3-K. In this regard, caveolin-1 appears to serve a scaffold forming a large signalplex, which includes AT1 receptor, EGF receptor and other signaling molecules such as Rac1 and NAD(P)H oxidase (Olivares-Reyes et al., 2005).
Given that βPIX-Rac1 activates PAK1 and NAD(P)H oxidase-ROS generation at the caveolae following Ang II stimulation (Figure 4), how are these two downstream effector pathways differentially activated? βPIX forms a multimeric complex with Rac1, PAK1 and GIT. βPIX was originally identified as a binding partner of PAK1 whose unconventional proline-rich motif (PXXXP) is involved (Manser et al., 1998). βPIX can constitutively interact with Rac1 and GIT through the same GIT binding domain (Shin et al., 2006). The SH3 domain of βPIX can interact with Rac1 (ten Klooster et al., 2006). Thus, formation of a multimeric complex containing βPIX, Rac1 and PAK1 may represent a molecular mechanism warranting specific PAK1 activation in this compartment. Consistent with this, depletion of βPIX prevented Ang II-stimulated PAK1 activation (Figure 4A). Similarly, a fraction of βPIX-Rac1 is considered to form a complex with NAD(P)H oxidase for ROS generation in an Ang II-dependent manner. When activated and translocated to the caveolae following Ang II stimulation (Zuo et al., 2004, 2005), active GTP-bound Rac1 interacts with p67phox/its homolog Noxa1 (Cheng et al., 2006; Miyano et al., 2006; Ueyama et al., 2006). This binding may induce conformational changes in p67phox/Noxa1, which, in turn, stimulates ROS generation by Nox2/Nox1 NAD(P)H oxidases expressed in VSMCs. Furthermore, βPIX can directly interact with Nox1 (Park et al., 2004). Collectively, the data support the notion that through specific protein-protein interaction βPIX-Rac1 may specifically activate downstream PAK1 and NAD(P)H oxidase.
βPIX-Rac1-PAK1 and βPIX-Rac1-NAD(P)H oxidase pathways are also differentially regulated by their upstream kinases. Of PKC, Src and PI3-K, only Src functions upstream of PAK1 activation (Figure 3B and 5). c-Src kinase phosphorylates the Rac1-sepcific exchange factor Tiam-1 (Servitja et al., 2003; Ma et al., 2008;), although it remains to be confirmed in VSMCs. βPIX cycles between the cytosol and focal adhesions, where it transiently forms a complex with paxillin (Rosenberger et al., 2006). It is well known that paxillin is an excellent substrate of c-Src (Weng et al., 1993). It is plausible that c-Src may also phosphorylate βPIX and modulate its GEF activity. Otherwise, Src may indirectly regulate βPIX via FAK, as FAK tyrosine phosphorylates βPIX (Chang et al., 2007). Recent observations suggest an alternative Rac/Cdc42-independent βPIX-PAK1 pathway for migration and morphological changes (Lucanic and Cheng, 2008). Existence of a ROS-dependent c-Src-PDK1-PAK1 pathway may explain the Rac1/Cdc42-independent mode of PAK1 activation (Weber et al., 2004). GF109236X-resistent but rottlerin-sensitive PKCδ can also bypass the βPIX-Rac1 pathway and activate PAK1 (Woolfolk et al., 2005). In contrast to the specific involvement of Src in activation of βPIX-PAK1 pathway, all three kinases presently examined lay upstream of ROS generation (Figure 3C and 5) consistent with a previous result (Seshiah et al., 2002).
Schematic diagram of the signaling pathway for Ang II-stimulated VSMC migration. Ang II stimulates VSMC migration through activation of multiple kinases such as Src, PKC and PI3-K. These kinases function upstream of βPIX-Rac1 pathway which sequentially activates PAK1 and NAD(P)H oxidase leading to ROS production. In the present study we have demonstrated that βPIX-dependent Rac1 activation is critically involved in the Ang II signaling. Regarding PAK1 activation, only Src is considered to function upstream of βPIX-Rac1 (Figure 3B). Src can activate PAK1 via PDK1 in a βPIX-Rac1 independent manner (not shown) (Weber et al., 2004). Although ROS is produced via Src-βPIX-Rac1-NAD(P)H oxidase, it can amplify its production through recycling a positive feedback loop. Thus, ROS generation can be sustained for several hours, which explains the mechanism for Ang II-stimulated VSMC migration.
The collective results support the following model for the signaling pathway in Ang II-stimulated migration of VSMC (Figure 5). Ang II stimulates multiple downstream kinases such as Src, PKC and PI3-K through the AT1 receptor. They sequentially activate the βPIX-Rac1 pathway, which differentially uses PAK1 and NAD(P)H oxidase-ROS depending on the upstream kinase. PAK1 and NAD(P)H oxidase-generated ROS promotes cell migration through cytoskeletal remodeling. We have focused on elucidating the role for this pathway in correlation with VSMC migration. Therefore, together with previous results showing that VSMC proliferation and hypertrophy also utilizes this pathway, its regulation might take center stage in Ang II-induced pathophysiology. It is also of note that PAK1 and released ROS may feed-forward βPIX-Rac1, which would explain the sustained activation of Rac1 activation (Seshiah et al., 2002).
A large body of evidence indicates that excessive activation of Ang II signaling contributes to the pathogenesis of cardiovascular diseases including coronary atherosclerosis, restenosis and hypertension. Ang II stimulates VSMC migration from media to intima during the development of atherosclerosis, thereby accelerating atheroma formation. Moreover, Ang II with other cytokines and growth factors facilitates transition of VSMCs from a contractile to synthetic phenotype, which induces proliferative responses in VSMCs. We demonstrate here that βPIX-Rac1 function as upstream regulators of PAK1 and NAD(P)H oxidase-ROS generation, which are essential for mediating Ang II-stimulated VSMC migration. The critical involvement of Rac1-NAD(P)H oxidase in cardiac hypertrophy highlights the importance of this pathway (Satoh et al., 2006). In conjunction with previous results, the present data deepen our understanding of the pathogenesis of cardiovascular diseases by defining the role for βPIX exchange factor.
Methods
Materials
Ang II, GTPγS, PP2, tetramethylrhodamine isothiocyanate (TRITC)-conjugated phalloidin, N-acetyl-1-cysteine and GF 109203X were purchased from Sigma-Aldrich (St. Louis, MO). LY294002 and NSC23766 were obtained from Calbiochem (LaJolla, CA). Lipofectamine 2000, siRNA for βPIX and FBS were purchased from Invitrogen (Carlsbad, CA). 2',7'-Dichlorofluorescein diacetate (DCF-DA) was obtained from Molecular Probes (Eugene, OR). Anti-phospho PAK, ERK1/2, JNK1/2 and PAK antibodies were purchased from Cell Signaling Technology (Danvers, MA).
Cell culture
VSMCs were collected by collagenase digestion of aortas from 7-week-old Sprague-Dawley rats as previously described (Chamley-Campbell et al., 1979). VSMCs were maintained in DMEM supplemented with 10% FBS and 1 × antibiotics (Invitrogen) at 37℃ in 5% CO2. Cells were passaged 6-7 times prior to being used for experiments.
siRNA transfection
Cells were seeded onto 60 mm-diameter culture dishes. A mixture of 100 nM siRNA and 5 µl Lipofectamine 2000 (Invitrogen) was added to culture dishes according to the manufacturer's instructions. After 48-72 h, transfected cells were analyzed by Western blotting with anti-βPIX antibody. βPIX-specific siRNA (5'GGAGGATTATCATCCTGATAG) and scrambled siRNA for control (Bioneer, Korea) were used.
Preparation of membrane fractions
VSMCs were incubated in hypotonic buffer (10 mM HEPES pH 7.4, 100 mM NaCl, 10% glycerol, 1 mM EDTA) for 1 h, harvested, and homogenized with a 26 G syringe. Cell lysates were precleared by centrifugation at 10,000 × g for 10 min. The supernatants were centrifuged at 100,000 × g for 30 min. After centrifugation, the pellet was solubilized using lysis buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 1% Triton X-100, 20 mM β-glycerophosphate, 1 mM sodium orthovanadate, 2 % n-octyl-β-D-glucoside) and centrifuged again at 100,000 × g for 30 min. The supernatant containing the membrane fraction was collected.
Immunoprecipitation and immunoblotting
Cells were lysed in lysis buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 100 mM NaF, 10% glycerol, 1% Triton X-100, 200 µM orthovanadate, 1 mM PMSF and a protease inhibitor cocktail) for 1 h at 4℃. Cell lysates were immunoprecipitated with PIX antibody for 4 h at 4℃. Immunoprecipitates were collected by adding protein G agarose, and washed five times with lysis buffer. Samples were fractionated by 12% SDS-PAGE, and transferred to a PVDF membrane in a Tris-glycine-methanol buffer (25 mM Tris base, 200 mM glycine, 20% methanol). Membranes were blocked with 3% skimmed milk in PBS for 30 min, incubated with primary antibodies for 1 h at room temperature (RT), and washed three times with PBS containing 0.1% Tween-20. Membranes were blotted with secondary HRP-conjugated antibodies for 1 h at RT. After five washes with PBS and 0.1% Tween-20, signals were detected using enhanced chemiluminescence reagent (Amersham Biosciences, Piscataway, NJ).
Guanine nucleotide exchange (GEF) assay
Activity of βPIX GEF was measured as previously described (Shin et al., 2004). Cells were pre-treated with PP2, GF 109203X or LY294002 for 1 h and stimulated with Ang II. Cell lysates were immunoprecipitated with anti-βPIX antibody. Immunoprecipitates were loaded with 100 µM GTPγs at 30℃ for 30 min in exchange buffer (20 mM HEPES, pH 7.5, 1 mM EDTA, 1 mM dithiotheitol, 50 mM NaCl) and washed three times with lysis buffer as described above. Immunoprecipitates were further incubated with purified GST-p21-binding domain (PBD) at 30℃ for 1 h in binding buffer (25 mM Tris HCl, pH 7.5, 1 mM dithiothreitol, 30 mM MgCl2, 40 mM NaCl, 0.5% Triton X-100) and washed three times with binding buffer. Beads were resolved by 12% SDS-PAGE and immunoblotted with anti-GST antibody. GST-PBD was expressed in Escherichia coli DH5α and purified with gluthathione-Sepharose affinity chromatography.
Wound migration assay
Cells were cultured to more than 90% confluence in a six-well plate. To produce the wound area, the confluent growth was scratched using end of a yellow tip across the center of the well. Cells were incubated under the indicated experimental conditions. At 12 h following migration, 10 randomly selected fields at the edge of the wound were photographed using an IX81-ZDC inverted microscope (Olympus Optical, Tokyo, Japan) equipped with a cooled Cascade 512B CCD camera (Photometrics, Tucson, AZ), and analyzed by MetaMorph software version 7.1.7 (Universal Imaging, Dowington, PA).
Measurement of ROS production
Cells were starved with serum free DMEM for 16 h and stimulated with 100 nM Ang II for 10 min. They were then washed with Hank's balanced salt solution (HSSB), incubated with 1 µM DCF-DA (Molecular Probes) for 10 min at 37℃ in the dark and washed twice with HSSB. Oxidation of fluorescent DCF dyes by released ROS was examined using an IX81-ZDC inverted fluorescence microscope (Olympus) and digitized using a Cascade 512B CCD camera. Images were analyzed by MetaMorph software. The fluorescence of 10 randomly selected fields was measured at each experiment.
Fluorescence microscopy
VSMCs were plated onto glass coverslips and treated with Ang II. Cells were fixed with 3.7% paraformaldeyde in PBS for 15 min, permeabilized with 0.2% Triton X-100 in PBS and blocked with a solution of 2% BSA and 2% FBS in PBS before staining. To localize Rac1, cells were stained with anti-Rac1 antibody (BD Bioscience, Franklin Lakes, NJ) followed by Alexa fluor 488-conjugated rabbit anti-mouse IgG antibody. TRITC-conjugated phalloidin was used for actin staining. After staining, coverslips were mounted onto a glass slide with a mounting gel. Cells were observed and photographed as described above in the wound migration assay.
Abbreviations
- Ang II:
-
angiotensin II
- GEF:
-
guanine nucleotide exchange factor
- PAK:
-
p21-activated kinase
- PBD:
-
p21-binding domain
- PI3-K:
-
phosphatidylinositol 3-kinase
- ROS:
-
reactive oxygen species
- VSMC:
-
vascular smooth muscle cell
References
Cau J, Hall A . Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways . J Cell Sci 2005 ; 118 : 2579 - 2587
Chamley-Campbell J, Campbell GR, Ross R . The smooth muscle cell in culture . Physiol Rev 1979 ; 59 : 1 - 61
Chang F, Lemmon CA, Park D, Romer LH . FAK potentiates Rac1 activation and localization to matrix adhesion sites: a role for betaPIX . Mol Biol Cell 2007 ; 18 : 253 - 264
Cheng G, Diebold BA, Hughes Y, Lambeth JD . Nox1-dependent reactive oxygen generation is regulated by Rac1 . J Biol Chem 2006 ; 281 : 17718 - 17726
del Pozo MA, Price LS, Alderson NB, Ren XD, Schwartz MA . Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK . Embo J 2000 ; 19 : 2008 - 2014
del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA . Integrins regulate Rac targeting by internalization of membrane domains . Science 2004 ; 303 : 839 - 842
Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y . Rational design and characterization of a Rac GTPase-specific small molecule inhibitor . Proc Natl Acad Sci USA 2004 ; 101 : 7618 - 7623
Hall A . Rho GTPases and the actin cytoskeleton . Science 1998 ; 279 : 509 - 514
Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S . Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology . Clin Sci (Lond) 2007 ; 112 : 417 - 428
Kim S, Kim T, Lee D, Park SH, Kim H, Park D . Molecular cloning of neuronally expressed mouse betaPix isoforms . Biochem Biophys Res Commun 2000 ; 272 : 721 - 725
Lucanic M, Cheng HJ . A RAC/CDC-42-independent GIT/PIX/PAK signaling pathway mediates cell migration in C. elegans . PLoS Genet 2008 ; 4 : e1000269 -
Lyle AN, Griendling KK . Modulation of vascular smooth muscle signaling by reactive oxygen species . Physiology (Bethesda) 2006 ; 21 : 269 - 280
Ma QL, Yang F, Calon F, Ubeda OJ, Hansen JE, Weisbart RH, Beech W, Frautschy SA, Cole GM . p21-activated kinase-aberrant activation and translocation in Alzheimer disease pathogenesis . J Biol Chem 2008 ; 283 : 14132 - 14143
Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Leung T, Lim L . PAK kinases are directly coupled to the PIX family of nucleotide exchange factors . Mol Cell 1998 ; 1 : 183 - 192
Mehta PK, Griendling KK . Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system . Am J Physiol Cell Physiol 2007 ; 292 : C82 - C97
Miyano K, Ueno N, Takeya R, Sumimoto H . Direct involvement of the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1 . J Biol Chem 2006 ; 281 : 21857 - 21868
Nola S, Sebbagh M, Marchetto S, Osmani N, Nourry C, Audebert S, Navarro C, Rachel R, Montcouquiol M, Sans N, Etienne-Manneville S, Borg JP, Santoni MJ . Scrib regulates PAK activity during the cell migration process . Hum Mol Genet 2008 ; 17 : 3552 - 3565
Ohtsu H, Mifune M, Frank GD, Saito S, Inagami T, Kim-Mitsuyama S, Takuwa Y, Sasaki T, Rothstein JD, Suzuki H, Nakashima H, Woolfolk EA, Motley ED, Eguchi S . Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II . Arterioscler Thromb Vasc Biol 2005 ; 25 : 1831 - 1836
Olivares-Reyes JA, Shah BH, Hernandez-Aranda J, Garcia-Caballero A, Farshori MP, Garcia-Sainz JA, Catt KJ . Agonist-induced interactions between angiotensin AT1 and epidermal growth factor receptors . Mol Pharmacol 2005 ; 68 : 356 - 364
Park HS, Lee SH, Park D, Lee JS, Ryu SH, Lee WJ, Rhee SG, Bae YS . Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2 . Mol Cell Biol 2004 ; 24 : 4384 - 4394
Ridley AJ . Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking . Trends Cell Biol 2006 ; 16 : 522 - 529
Rosenberger G, Kutsche K . AlphaPIX and betaPIX and their role in focal adhesion formation . Eur J Cell Biol 2006 ; 85 : 265 - 274
Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK . Requirement of Rac1 in the development of cardiac hypertrophy . Proc Natl Acad Sci USA 2006 ; 103 : 7432 - 7437
Schmitz U, Thommes K, Beier I, Wagner W, Sachinidis A, Dusing R, Vetter H . Angiotensin II-induced stimulation of p21-activated kinase and c-Jun NH2-terminal kinase is mediated by Rac1 and Nck . J Biol Chem 2001 ; 276 : 22003 - 22010
Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshornae DJ, Nakano T . Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle . Circ Res 2003 ; 92 : 411 - 418
Servitja JM, Marinissen MJ, Sodhi A, Bustelo XR, Gutkind JS . Rac1 function is required for Src-induced transformation. Evidence of a role for Tiam1 and Vav2 in Rac activation by Src . J Biol Chem 2003 ; 278 : 34339 - 34346
Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK . Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators . Circ Res 2002 ; 91 : 406 - 413
Shin EY, Shin KS, Lee CS, Woo KN, Quan SH, Soung NK, Kim YG, Cha CI, Kim SR, Park D, Bokoch GM, Kim EG . Phosphorylation of p85 beta PIX, a Rac/Cdc42-specific guanine nucleotide exchange factor, via the Ras/ERK/PAK2 pathway is required for basic fibroblast growth factor-induced neurite outgrowth . J Biol Chem 2002 ; 277 : 44417 - 44430
Shin EY, Woo KN, Lee CS, Koo SH, Kim YG, Kim WJ, Bae CD, Chang SI, Kim EG . Basic fibroblast growth factor stimulates activation of Rac1 through a p85 betaPIX phosphorylation-dependent pathway . J Biol Chem 2004 ; 279 : 1994 - 2004
Shin EY, Lee CS, Cho TG, Kim YG, Song S, Juhnn YS, Park SC, Manser E, Kim EG . BetaPak-interacting exchange factor-mediated Rac1 activation requires smgGDS guanine nucleotide exchange factor in basic fibroblast growth factor-induced neurite outgrowth . J Biol Chem 2006 ; 281 : 35954 - 35964
ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL . Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix . J Cell Biol 2006 ; 172 : 759 - 769
Ueyama T, Geiszt M, Leto TL . Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases . Mol Cell Biol 2006 ; 26 : 2160 - 2174
Volinsky N, Gantman A, Yablonski D . A Pak- and Pix-dependent branch of the SDF-1alpha signalling pathway mediates T cell chemotaxis across restrictive barriers . Biochem J 2006 ; 397 : 213 - 222
Wakino S, Hayashi K, Kanda T, Tatematsu S, Homma K, Yoshioka K, Takamatsu I, Saruta T . Peroxisome proliferator-activated receptor gamma ligands inhibit Rho/Rho kinase pathway by inducing protein tyrosine phosphatase SHP-2 . Circ Res 2004 ; 95 : e45 - e55
Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK . Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration . Circ Res 2004 ; 94 : 1219 - 1226
Weng Z, Taylor JA, Turner CE, Brugge JS, Seidel-Dugan C . Detection of Src homology 3-binding proteins, including paxillin, in normal and v-Src-transformed Balb/c 3T3 cells . J Biol Chem 1993 ; 268 : 14956 - 14963
Woolfolk EA, Eguchi S, Ohtsu H, Nakashima H, Ueno H, Gerthoffer WT, Motley ED . Angiotensin II-induced activation of p21-activated kinase 1 requires Ca2+ and protein kinase CΔ in vascular smooth muscle cells . Am J Physiol Cell Physiol 2005 ; 289 : C1286 - C1294
Wyse BD, Prior IA, Qian H, Morrow IC, Nixon S, Muncke C, Muncke C, Kurzchalia TV, Thomas WG, Parton RG, Hancock JF . Caveolin interacts with the angiotensin II type 1 receptor during exocytic transport but not at the plasma membrane . J Biol Chem 2003 ; 278 : 23738 - 23746
Za L, Albertinazzi C, Paris S, Gagliani M, Tacchetti C, de Curtis I . betaPIX controls cell motility and neurite extension by regulating the distribution of GIT1 . J Cell Sci 2006 ; 119 : 2654 - 2666
Zenke FT, King CC, Bohl BP, Bokoch GM . Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity . J Biol Chem 1999 ; 274 : 32565 - 32573
Zheng Y, Im CN, Seo JS . Inhibitory effect of Hsp70 on angiotensin II-induced vascular smooth muscle cell hypertrophy . Exp Mol Med 2006 ; 38 : 509 - 518
Zuo L, Ushio-Fukai M, Hilenski LL, Alexander RW . Microtubules regulate angiotensin II type 1 receptor and Rac1 localization in caveolae/lipid rafts: role in redox signaling . Arterioscler Thromb Vasc Biol 2004 ; 24 : 1223 - 1228
Zuo L, Ushio-Fukai M, Ikeda S, Hilenski L, Patrushev N, Alexander RW . Caveolin-1 is essential for activation of Rac1 and NAD(P)H oxidase after angiotensin II type 1 receptor stimulation in vascular smooth muscle cells: role in redox signaling and vascular hypertrophy . Arterioscler Thromb Vasc Biol 2005 ; 25 : 1824 - 1830
Acknowledgements
This work was supported, in part, by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (R13-2007-001-01001-0 (2009)), Grants R01-2005-000-10386-0 (2007) through the Basic Research Program from the Korean Science and Engineering Foundation (KOSEF) and by the Korea Research Foundation Grant (KRF-2003-003-E00025).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Shin, EY., Lee, CS., Park, MH. et al. Involvement of βPIX in angiotensin II-induced migration of vascular smooth muscle cells. Exp Mol Med 41, 387–396 (2009). https://doi.org/10.3858/emm.2009.41.6.044
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2009.41.6.044
Keywords
This article is cited by
-
Cytoskeletal Regulation of TRPC Channels in the Cardiorenal System
Current Hypertension Reports (2012)