Abstract
In this study, we investigated the role of Nur77, an orphan nuclear receptor, in HIF-α transcriptional activity. We found that Nur77 associates and stabilizes HIF-1α via indirect interaction. Nur77 was found to interact with pVHL in vivo via the α-domain of pVHL. By binding to pVHL, Nur77 competed with elongin C for pVHL binding. Moreover, Nur77-binding to pVHL inhibited the pVHL-mediated ubiquitination of HIF-1α and ultimately increased the stability and transcriptional activity of HIF-1α. The ligand-binding domain of Nur77 was found to interact with pVHL and the expression of this ligand-binding domain was sufficient to stabilize and transactivate HIF-1α. Under the conditions that cobalt chloride was treated or pVHL was knocked down, Nur77 could not stabilize HIF-α. Moreover, Nur77 could not further stabilize HIF-2α in A498/VHL stable cells, which is consistent with our finding that Nur77 indirectly stabilizes HIF-α by binding to pVHL. Thus, our results suggest that an orphan nuclear receptor Nur77 binds to pVHL, thereby stabilizes and increases HIF-α transcriptional activity under the non-hypoxic conditions.
Similar content being viewed by others
Introduction
Hypoxia is defined as a condition of limited cellular oxygen tension. Cells under hypoxia adapt by expressing essential genes for energy metabolism, erythropoiesis, and angiogenesis (Semenza, 2001; Wenger, 2002). Hypoxia inducible factor (HIF) is a key transcription factor for the genes induced under hypoxic conditions and is composed of an oxygen-regulated α subunit and a constitutively expressed β ubunit, the latter of which is known as aryl hydrocarbon receptor nuclear translocator (ARNT). Under hypoxia, the α subunit of HIF is translocated into the nucleus and forms a heterodimer with the β subunit, which binds to HIF responsive element (HRE) and transactivates target genes.
von Hippel-Lindau (VHL), a tumor suppressor, is the causative gene of VHL associated diseases, such as hemangioblastomas, renal cell carcinomas, and pheochromocytomas (Safran and Kaelin, 2003). pVHL, which resembles SCF complex in yeast, forms a multimeric complex containing elongin C, elongin B, Cul2 and Rbx1, that degrades target proteins (Stebbins et al., 1999; Ivan et al., 2001; Jaakkola et al., 2001; Safran and Kaelin, 2003). pVHL has two domains, the α domain which recruits elongin C/elongin B/Cul2/Rbx1 complex, and the β domain which can interact with the hydroxylated oxygen-dependent degradation (ODD) domain of HIF-1α (Ivan et al., 2001; Jaakkola et al., 2001). In general, oxygen-sensing HIF-prolyl-hydroxylases hydroxylate the proline residue at the conserved sequence (LXXLAP) within an ODD domain of HIF-1α. This hydroxylated HIF-1α protein can interact with pVHL, and is then ubiquitinylated, and rapidly degraded by 26S proteasomes (Semenza, 2001; Safran and Kaelin, 2003). In addition to the hypoxic stabilization of HIF-1α by the inhibition of its hydroxylation, non-hypoxic stimuli have been reported to increase the expression of HIF-1α under normoxic conditions (Hellwig-Burgel et al., 1999; Chen et al., 2001; Fukuda et al., 2002; Jung et al., 2003). Although their mechanisms are not fully understood, these processes focus on transcriptional or translational increases in HIF-1α expression (Zhong et al., 2000; Laughner et al., 2001; Page et al., 2002; Zhou et al., 2004).
Nur77, an orphan nuclear receptor, plays an important role in apoptosis in immature T cell, T cell hybridoma, and cancer cells (Li et al., 1998, 2000; Winoto and Littman, 2002; Wilson et al., 2003; Lin et al., 2004; Kim et al., 2005a). In addition, there are evidences to show that survival signals can induce Nur77. Nur77 was rapidly induced by several growth factors and mitogens (Hazel et al., 1988; Williams and Lau, 1993; Yoon and Lau, 1994; Katagiri et al., 2000; Kolluri et al., 2003), indicating that Nur77 is at least involved in the proliferations of specific cell types. In addition, we recently showed that HIF-1 specifically induced Nur77 in VHL-deficient renal cell carcinoma (Choi et al., 2004). Moreover, independently, HIF was reported to induce Nur77 in several cancer cell types (Yoo et al., 2004). Summarizing, the above suggest that Nur77 cooperates with HIF and participates in cancer cell proliferation or apoptosis.
In this study, we identified the mechanism whereby Nur77 stabilizes and enhances HIF transcriptional activity. Nur77 was found to specifically bind to the α-domain of pVHL, and to protect HIF from pVHL-elongin C complex-mediated degradation. Therefore, our findings suggest that Nur77 has a potential role in regulating HIF transcription factor in cancer cell proliferation or apoptosis.
Materials and Methods
Cell culture and transfection
HEK293, SK-N-SH (neuroblastoma), A498 (renal cell carcinoma) and PC12 (pheochromocytoma) were purchased from ATCC and grown in DMEM supplemented with 10% FBS and 50 U/ml streptomycin/penicillin. Cells were transfected with Lipofectamine reagent (Invitrogen) according to the manufacturer's directions.
Plasmid constructs
pCMV-HA-HIF-1α and pCR3-VHL-HA were prepared as described previously (Choi et al., 2004). HA-tagged proline mutated HIF-1α [P402A, P564A] were made using Quick-Change Site-directed Mutagenesis kit (Stratagene). DNA constructs of full-length and truncated mutants of Nur77 were generated by subcloning PCR products into pCS2+MT vector. Mammalian expression vector encoding flag-tagged Nurr1 was generously provided by Dr. Lee (Hanyang University, Korea). Myc-tagged Nor-1 was constructed by cloning PCR product of human Nor-1 cDNA, which was provided by Dr. Ohkura (National Cancer Center Research Institute, Japan), into pCS2+MT vector. DNA constructs encoding the bacterially-expressing GST-fused full-length, α or β domains of pVHL proteins (pGEX-5X-1-pVHL, pGEX-5X-1-pVHL-α, pGEX-5X-1-pVHL-β) and mammalian expression vectors for myc-Gal4-elongin C (pcDNA3.1-myc-Gal4-elongin C) were described previously (Roe et al., 2006). The luciferase reporter plasmid, 3 × HRE_tk_luciferase, was generated by subcloning three copies of EPO-HRE (TACGTGCT) [one copy covers 50 bp long sequences (from + 2,830 to + 2,880) of human erythropoietin (EPO) promoter] sequences and herpes simplex virus thymidine kinase promoter (from -105 to + 57) into pGL2 basic vector. Detailed information about the plasmids used in this study is available upon request.
Immunoprecipitation, immunoblotting, and GST-pull down assay
Transiently transfected HEK293 cells were harvested after 24 h and lysed with lysis buffer [20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.5% NP-40 and 1 mM PMSF]. Whole cell extracts were obtained by sonicating cell lysates, and immunoprecipitated using suitable antibodies and Protein A/G coated beads (Santa Cruz Biotechnology). For GST-pulldown, cell lysates were precipitated with GST or GST fusion proteins in the presence of GSH-beads (Amersham Pharmacia). Immunoprecipitates were separated by SDS-PAGE, electrotransferred to nitrocellulose membrane, and immunoblotted with appropriate antibodies. Anti-HIF-1α was purchased from BD Transduction Laboratory; anti-HIF-2α from Novus; anti-lamin B, and anti-ubiquitin from Santa Cruz Biotechnology; anti-Flag(M2) and anti-β-actin from Sigma; anti-HA, and anti-myc from Covance.
Immunofluorescent staining
HEK293 cells were transiently transfected with pCS2+MT-Nur77 and/or pCMV-HA-HIF-1α. Cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton-X-100. After blocking with 2% BSA, the cells were stained with anti-Nur77 antibody (Santa Cruz Biotechnology) and anti-HA antibody (Covance). The cells were then washed with ice-chilled PBS, and stained with FITC-conjugated anti-rabbit and Rhodamine-conjugated anti-mouse antibodies (Jackson Immuno Research). Cells were analyzed using an LSM PASCAL 5 (Zeiss) confocal microscope system.
RT-PCR
Total cellular RNA was extracted from cells using TRIzol reagent (Invitrogen) as per the manufacture's protocol. cDNA was made from total RNAs using a cDNA synthesis kit (Takara), and PCR was performed under standard conditions for 30 cycles. The primer pairs used were as follows: sense 5'-CTCAAAGTCGGACAGCCTCA-3' and antisense 5'-CCCTGCAGTAGGTTTCTGCT-3' for HIF-1α ; sense 5'-CTCAAAGTCGGACAGCCTCA-3' and antisense 5'-CACGGACTGATGCGGAGGGAG-3' for HA-HIF-1α ; sense 5'-GACGAATTCATGTCCGAGAACGGAGCGCCCGGG-3' and antisense 5'-GATCTCGAGTCAAAAGGTGCTGGTGGAGGTTG T-3' for BNIP3; sense 5'-GATGTCGTCCCACCTAGTCGA G-3' and antisense 5'-CTTCTGACTGAGAGCTATGGTCCC-3' for BNIP3-like; sense 5'-CAACTGGACCTCAAATTTCATTGTGGG-3' and antisense 5'-CGGGTGTCTTATCACTTTGGCTGG-3' for Glut1; sense 5'-GGCATCCACGAAACTACCTT-3' and antisense 5'-CTGTGTGGACTTGGGAGAGG-3' for β-actin.
Proteasome inhibition and in vivo ubiquitination assay
HEK293 cells were transiently transfected with mammalian expression vectors for (myc)6-Nur77, HA-HIF-1α, pVHL-HA, and HA-Ub. After 24 h of incubation, cells were treated with MG132 (15 µM; Calbiochem) for a further 5 h. Cells were lysed with lysis buffer and immunoprecipitated with anti-HIF-1α antibody (Santa Cruz Biotechnology). Immunoprecipitates were subjected to 6% SDS-PAGE, and ubiquitinylated HIF-1α was detected with anti-ubiquitin antibody (Santa Cruz Biotechnology).
RNA interference
The siRNAs for VHL and GFP were chemically synthesized by Dharmacon. siVHL corresponds to positions 609-629 of the coding region (Berra et al., 2003). As a control, siGFP was used (5'-GGCUACGUCCAGGAGCGCACCUU-3', 5'-GGUGCGCUCCUGGACGUAGCCUU-3').
Cotransfection plasmid DNA and RNAi into HEK293 cells
Mammalian expression vectors for pCMV-HA-HIF-1α and/or pCS2+MT-Nur77 (each 0.5 µg) were mixed with total 50 pmol of either siVHL or siGFP. The total amount of expression vectors used in this study was adjusted to 1.0 µg using each empty vector. The mixture was treated into HEK293 cells using Lipofectamine 2000 reagents (Invitrogen).
Retroviral infection
Myc-tagged Nur77 was made by cloning PCR product into pMT retroviral vector, which was generously provided by Dr. Yu (Seoul National University, Korea). pBabe-HA-tagged pVHL was made as described previously (Roe et al., 2006) For the production of retrovirus, HEK293T cells were transfected in 100 mm dishes using Retrovirus Packaging Kit (Takara). Medium was changed 5 h after transfection and further incubated for 48 h. Culture supernatants were harvested and filtered using 0.22 µM filter. For retroviral infection, retroviral supernatants, which were diluted with fresh medium at ratio of 1 : 1, were added to A498 cells (105 cells in 35 mm dish) and incubated for 10 h in the presence of polybrene (8 µg/ml). After changing medium, cells were further incubated for 24 h. At next day, retroviral infection was repeated once more. A498/pBabe and A498/pVHL-HA cells, stably expressing HA-tagged pVHL, were established by selection of puromycin-resistant cells after retroviral infection of A498 cells. pMT-GFP vector was used as a control of retroviral infection.
Statistics
Data are presented as means ± SDs and were analyzed by one-way ANOVA test. A P-value of less than 0.05 was considered statistically significant.
Results
Nur77 associates with HIF-1α and increases HIF-1α stabilization
To test the functional correlation between Nur77 and HIF-1α, we first examined the physical interaction between myc-Nur77 and HA-HIF-1α. HEK293 cell lysates overexpressing myc-Nur77 and/or HA-HIF-1α were immunoprecipitated with anti-HA antibody, and then these immunoprecipitates were subjected to western blotting with anti-myc antibodies. It was found that HA-HIF-1α co-precipitated with myc-Nur77 (Figure 1A). We further confirmed the colocalization of HA-HIF-1α and myc-Nur77 at the single cell level by immunofluorescent staining and confocal microscopy, which showed that both proteins were localized in the nucleus (Figure 1B). Intriguingly, we observed that, when HA-HIF-1α was coexpressed with myc-Nur77, protein content of HA-HIF-1α was markedly increased in western blots (Figure 1A, input lane 3) and at the single cell level (Figure 1B). To determine whether Nur77 increases the stabilization of HIF-1α, a fixed amount of HA-HIF-1α expression vector was transiently transfected along with increasing amounts of myc-Nur77 expression vectors. As expected, a gradual increase in myc-Nur77 led to HA-HIF-1α stabilization (Figure 2A). However, the mRNA level of endogenous and transfected HIF-1α did not vary, indicating that Nur77 modulates HIF-1α at the protein level (Figure 2B). Next, we examined whether expression of Nur77 would affect endogenous level of HIF-1α protein. When nuclear extracts from HEK293 cells transfected with myc-tagged Nur77 or CoCl2-treated HEK293 cells were immunoblotted with anti-HIF-1α antibody, Nur77 expression led to stabilize an endogenous HIF-1α just like the treatment of CoCl2 (Figure 2C). We next tested whether Nur77-mediated HIF-1α stabilization is linked to HIF-1α transcriptional activity. When two types of cells (SK-N-SH and PC-12) were transfected with increasing amount of Nur77 in the presence of HIF-1α and 3 × HRE_tk_luciferase reporter gene, Nur77 was revealed to increase HIF-1α-dependent transcriptional activity (Figure 2D).
Nur77 interacts with and accumulates HIF-1α. (A) HEK293 cells were transiently transfected with pCMV-HA-HIF-1α and pCS2+MT-Nur77. Cell lysates were immunoprecipitated with anti-HA antibody, and probed with anti-myc antibody. (B) Nur77 colocalizes with and accumulates HIF-1α in nucleus. HEK293 cells were transiently transfected with the same expression vectors as in (A), stained with anti-HA and anti-Nur77 antibodies, and then treated with anti-mouse-IgG-rhodamine and anti-rabbit IgG-FITC antibodies. After extensive washing, cells were observed under a confocal microscope.
Nur77 overexpression stabilized HIF-1α protein and increased HIF-1α transcription activity. (A) Nur77 overexpression stabilized HIF-1α at the protein level. HEK293 cells were transiently transfected with pCMV-HA-HIF-1α (0.1 µg) and/or increasing amount of pCS2+MT-Nur77. After 24 h of incubation, cell lysates were subjected to SDS-PAGE, and then immunoblotted with either anti-HA or anti-myc antibody. The same amount of cell lysate was probed with anti-β-actin antibody as a control. (B) Nur77 did not affect the mRNA level of HIF-1α. Total cellular RNA was prepared from HEK293 cells transfected with the indicated constructs. RT-PCR was performed as described in Materials and Methods. (C) Nur77 induced endogenous HIF-1α. HEK293 cells were transfected with pCS2+MT-Nur77. After 24 h of incubation, nuclear extracts were prepared as described previously (Kim et al., 2005b). Nuclear extracts were subjected to SDS-PAGE, and then immunoblotted with HIF-1α antibody or anti-lamin B antibody. As a positive control for detecting endogenous HIF-1α, HEK293 cells were treated with 200 µM of CoCl2 for 5 h. (D) Nur77 enhanced the transactivation of HIF-1α. SK-N-SH or PC-12 cells were transfected with luciferase reporter gene (3 × HRE_tk_luciferase) driven by 3 × HRE [+2,830 - +2,880 of human erythropoietin (EPO) promoter] and HSV-tk promoter region (-105 - +57), pCMV-HA-HIF-1α, and /or increasing amounts of pCS2+MT-Nur77. After 24 h of incubation, reporter activities were measured in cell lysates. Reporter activities were normalized with respect to lysate protein concentration. Means ± SDs for n = 3 are shown. P < 0.01 versus control.
Nur77 inhibits the pVHL-mediated degradation of HIF-1α
We next investigated the direct interaction between Nur77 and HIF-1α using in vitro translated HIF-1α and GST-fused Nur77 protein. However, we failed to detect any direct interaction between the two molecules (data not shown). HIF-1α is mainly regulated at the protein level through the oxygen-dependent pVHL-mediated ubiquitination pathway, and subsequent rapid degradation (Safran and Kaelin, 2003). Therefore, we explored the possibility that Nur77 increased HIF-1α protein levels by inhibiting pVHL-mediated degradation. When myc-Nur77 was co-expressed with pVHL-HA and HA-HIF-1α, we found that Nur77 blocked the pVHL-mediated degradation of HIF-1α (Figure 3A).
Nur77 blocked the pVHL-mediated ubiquitination of HIF-1α. (A) HEK293 cells were transiently transfected with different combinations of pCS2+MT-Nur77, pCR3-VHL-HA, and pCMV-HA-HIF-1α. Protein expressions were detected using antibodies recognizing tagged sequences. This experiment is one representative of three independent experiments. (B) Nur77 reduced the ubiquitination of HIF-1α. HEK293 cells were transfected with the indicated constructs. After 24 h of incubation, cells were treated with MG132 (15 µM) (Calbiochem) for 5 h and then lysed. Cell lysates were immunoprecipitated with anti-HIF-1α antibody and then immunoblotted with anti-ubiquitin antibody (Santa Cruz Biotechnology). (C) Nur77 extends the half-life of HIF-1α. HEK293 cells were transiently transfected with pCMV-HA-HIF-1α with or without pCS2+MT-Nur77. After 24 h of incubation, cells were treated with cycloheximide (CHX, 25 µg/ml). Cell lysates were prepared at the shown time points, immunoprecipitated with anti-HA antibody, and immunoblotted with anti-HA antibody. The graph on the lower panel represents relative band intensities. Means ± SDs for n = 3 are shown. P < 0.01 versus control. Immunoblots represent one of three independent experiments.
We then examined whether Nur77 modulates the pVHL-mediated ubiquitination of HIF-1α. HEK293 cells were transiently transfected with different combinations of HA-ubiquitin, myc-Nur77, pVHLHA, and HA-HIF-1α. After 24 h of incubation, cells were treated with MG132 for 5 h. Each cell lysates was immunoprecipitated with anti-HIF-1α polyclonal antibody and probed with anti-ubiquitin antiantibody. The overexpression of myc-Nur77 was thus found to significantly reduce pVHL-mediated ubiquitination of HA-HIF-1α (Figure 3B, lane 4). Therefore, it is highly plausible that Nur77 extends the half-life of HIF-1α protein. As expected, Nur77 protected HIF-1α protein from degradation after protein synthesis abrogation by cycloheximide (Figure 3C). Given that pVHL is a specific E3 ligase of HIF-1α and that the ubiquitination of HIF-1α is reduced in the presence of Nur77, we conclude that Nur77 inhibits the pVHL-mediated ubiquitination of HIF-1α.
Nur77 binds to the α-domain of pVHL and competes with elongin C for pVHL binding
The finding that Nur77 inhibits HIF-1α ubiquitination implies that Nur77 may modulate HIF-1α stabilization by associating with pVHL. We thus examined the physical interaction between Nur77 and pVHL. HEK293 cells were transiently transfected with myc-Nur77, pVHL-HA, or both, and cell lysates were immunoprecipitated with anti-myc antibody. When immunoprecipitates were probed with anti-HA antibody, pVHL was detected only in the presence of both Nur77 and pVHL (Figure 4A). On the other hand, anti-HA antibody recognizing pVHL also immunoprecipitated Nur77, indicating that these proteins specifically associate with each other.
Nur77 binds to pVHL and competes with elongin C. (A) Nur77 interacts with pVHL. Cell lysates prepared from HEK293 cells transfected with pCR3-VHL-HA and/or pCS2+MT-Nur77, were immunoprecipitated and immunoblotted with appropriate antibodies as indicated in the figure. (B) Nur77 binding to the α subdomain of pVHL. Cell lysates were prepared from HEK293 cells transfected with pCS2+MT-Nur77 or pCMV-HA-HIF-1α and precipitated with GST-fused α (amino acids 155-213) or β (amino acids 2-154) domains of pVHL. Precipitates were probed with anti-myc or anti-HA antibodies. (C, D) Nur77 competed with elongin C for binding to pVHL. In (C), HEK293 cells were transiently transfected with pCR3-VHL-HA and pcDNA3-myc-Gal4-elongin C in the presence of increasing amounts of pCS2+MT-Nur77. Cell lysates were immunoprecipitated with anti-HA and elongin C was detected using anti-myc antibody. In (D), HEK293 cells were transiently transfected with pCR3-VHL-HA and pCS2+MT-Nur77 in the presence of increasing amounts of pcDNA3-myc-Gal4-elongin C. Graph data are expressed as the means ± SD for three independent experiments. Immunoblots represent one of three independent experiments. (E) Nur77 did not disrupt the pVHL-HIF interaction. HEK 293 cells were transiently transfected with pcDNA3-VHL-flag and pCMV-HA-HIF-1α in the presence of increasing amounts of pCS2+MT-Nur77. Cell lysates were immunoprecipitated with anti-flag antibody and HIF-1α was detected using anti-HA antibody.
Since we found that Nur77 binds to pVHL and inhibits the pVHL-mediated degradation of HIF-1α, we tried to identify the Nur77 binding domain on pVHL. It is known that pVHL is composed of two domains, the α and β domains (Stebbins et al., 1999). Thus, we generated two truncated mutants, GST-pVHL-α (155-213) and GST-pVHL-β (1-154), and performed GST-pull-down assays (Figure 4B). We first confirmed that HIF-1α protein interacted with the β domain of pVHL, which is consistent with previous result (Ohh et al., 2000; Ivan et al., 2001). However, we found that Nur77 interacted with the α-domain of pVHL (Figure 4B). Since elongin CB complex is known to bind to the α-domain of pVHL and serve as an ubiquitin ligase complex, we speculated that Nur77 competes with elongin C for binding to the α-domain of pVHL. To address this question, we examined the intensity of the interaction between pVHL-HA and myc-Gal4-elongin C on increasing the amount of Nur77 (Figure 4C). As expected, increasing Nur77 markedly reduced the interaction between pVHL and elongin C. Vice versa, when we examined the intensity of the interaction between pVHL-HA and myc-Nur77 on increasing the amount of myc-Gal4-elongin C, increasing amount of myc-Gal4-elongin C significantly reduced the interaction between pVHL and Nur77 (Figure 4D). When we examined whether myc-Nur77 affected the interaction between HA-HIF-1α and flag-pVHL, we found that myc-Nur77 did not interrupt the interaction between HA-HIF-1α and flag-pVHL (Figure 4E). On the contrary, increasing myc-Nur77 resulted in a strong interaction between HA-HIF-1α and flag-pVHL, and this was proportional to the Nur77-mediated stabilization of HIF-1α (Figure 4E). We concluded that Nur77 specifically binds to the α-domain of pVHL, which ultimately prevented pVHL-mediated degradation of HIF-1α.
pVHL binds to the ligand-binding domain of Nur77
Nur77 is composed of three domains, a transactivation domain (TAD), a DNA-binding domain (DBD), and a ligand-binding domain (LBD) (Figure 5A). To identify the pVHL-binding site in Nur77, we generated three truncation mutants and then coimmunoprecipitated them with pVHL. The results revealed that flag-pVHL binds to the myc-tagged ligand-binding domain of Nur77 (myc-Nur77-LBD), but not to other domains, indicating that Nur77-LBD is sufficient for pVHL binding (Figure 5B).
Binding of LBD-Nur77 to pVHL leads to stabilize and transactivate HIF-1α. (A) Schematic diagram of Nur77. (B) Mapping of the pVHL-binding site on Nur77. Three different pCS2+MT constructs of Nur77 truncated mutants (TAD; amino acid 1-265, DBD; amino acid 266-400, LBD; amino acid 401-601) and pCR3-VHL-HA were transiently transfected into HEK293 cells. Cell lysates were immunoprecipitated with anti-flag antibody and immunoblotted with anti-myc antibody. (C) LBD-Nur77 stabilizes HIF-1α and increases its transcriptional activity. HEK293 cells were transfected with different Nur77 truncated mutants along with 3 × HRE-tk-luciferase plasmid. HIF-1α stabilization was detected with anti-HA antibody, and Nur77 mutants were probed using anti-myc antibody. (D) Incremental increases in LBD enhanced HIF-1α transcriptional activity. The experimental conditions in (D) are the same as (C). In both (C) and (D), data are expressed as the mean ± SD for three independent experiments. Immunoblots represent one of three independent experiments. (E) The expression of endogenous BNIP3. The ectopic expression of LBD induces HIF-downstream genes. mRNAs were isolated from transfected HEK293 cells. The expressions of BNIP3, BNIP3-like, Glut1 and β-actin were detected by RT-PCR. The primers used in this experiment are described in Materials and Methods.
Nur77-LBD stabilizes and increases HIF-1α transcriptional activation
We next examined whether Nur77-LBD increased the HIF-1α transcriptional activity. When we transiently transfected HEK293 cells with expression vectors of myc-tagged Nur77 truncated mutants (FL, TAD, DBD, LBD) along with HA-HIF-1α and 3 × EPO-luciferase plasmid, we found that myc-Nur77-LBD stabilized HIF-1α and increased HIF-1α transcriptional activity as much as full-length Nur77 (Figure 5C). Moreover, a gradual increase in Nur77-LBD increased HIF-1α reporter activity (Figure 5D). When myc-Nur77-LBD was expressed, HIF-downstream genes (BNIP3, BNIP-like, Glut1) were increased (Figure 5E). Therefore, we concluded that Nur77-LBD alone is sufficient to stabilize and increase HIF-1α transcriptional activation.
pVHL is required for Nur77-mediated HIF-α stabilization
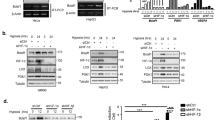
To understand the functional role of pVHL in Nur77-mediated HIF-1α stabilization, we transiently transfected HEK293 cells with expression vectors for HA-HIF-1α along with or without myc-Nur77. After 24 h of incubation, cells were treated with CoCl2 (200 µM) for further 5 h. As expected, either CoCl2-treatment or Nur77-overexpression could stabilize HIF-1α (Figure 6A, lane 2 and 3). Intriguingly, overexpressing myc-Nur77 did not further stabilize HIF-1α in the presence of CoCl2 (Figure 6A, lane 4), indicating that CoCl2 probably leads to the dissociation of pVHL with HIF-1α, and ultimately Nur77 can not be accessible to HIF-1α. We thus examined the effect of CoCl2 on the HIF-1α-Nur77 interaction. As expected, we confirmed that CoCl2 reduced the interaction affinity between Nur77 and HIF-1α (Figure 6B). Since the hydroxylation of two proline residues in HIF-1α is critical for pVHL-mediated degradation, we generated a pVHL-insensitive mutant, HIF-1α (P402A /P564A) and examined the Nur77-mediated stabilization of HIF-1α. As reported previously, this mutant was constitutively stabilized even in normoxic conditions. In addition, Nur77 could not further stabilize HIF-1α (P402A / P564A) mutant (Figure 6C).
pVHL is required for the Nur77-mediated HIF-1α stabilization. (A) The treatment of cobalt chloride abolishes the Nur77-mediated HIF-1α stabilization. HEK293 cells were transfected with HA-HIF-1α along with or without myc-Nur77. After 24 h of incubation, cells were treated with 200 µM of cobalt chloride for further 5 h. Cell lysates were immunoblotted with anti-HA and anti-myc antibodies for detecting HIF-1α and Nur77, respectively. β-actin was used as a control. (B) HEK293 cells transfected with indicated plasmids were incubated in the presence or absence of CoCl2. Cell lysates were immunoprecipitated with anti-myc antibody and immunoblotted with anti-HA antibody. (C) HEK293 cells were transfected with wild type or proline mutated (P402A/P564A) HIF-1α along with or without myc-Nur77. Cell lysates were immunoblotted with indicated antibodies. (D) Stable expression of HA-tagged VHL and endogenous expression of HIF-2α in established A498 cells was verified by immunoblot using specific antibodies. (E) Retrovirus expressing GFP (pMT-GFP), empty vector (pMT) or myc-tagged Nur77 was infected to A498/pBabe or A498/VHL stable cells as indicated. After 48 h of incubation, cell lysates were subjected to SDS-PAGE and immunoblotted with suitable antibodies.
We thus examined whether other homologues of Nur77, Nurr1 and Nor-1 could affect HIF-1α stabilization. Intriguingly, Nurr1, not Nor-1 could bind to pVHL just like Nur77 (Supplementary Figure 1A). Furthermore, Nurr1 could stabilize HIF-1α protein and increase the HIF-1α transcriptional activity (Supplementary Figure 1B and C). This finding implies that both Nur77 and Nurr1 could increase HIF-1α transcriptional activity through regulating binding between pVHL and HIF-1α. Vice versa, we next knocked down the endogenous pVHL and examined the Nur77-mediated HIF-1α stabilization. The treatment of siVHL reduced the 50% of mRNA level of endogenous VHL. In conditions that VHL level was reduced, the overexpression of Nur77 could not further increase HIF-1α stabilization (Supplementary Figure 2). We finally reconfirmed this finding in a VHL-negative tumor cell line. To this end, we first generated A498/pBabe and A498/VHL stable cells. A498/VHL stable cells were confirmed to destabilize HIF-2α, whereas A498/pBabe RCC cells constitutively stabilized HIF-2α (Figure 6D). We then infected retrovirally-expressed Nur77 into either A498/pBabe or A498/VHL and investigated the expression of HIF-2α in both cells. Since A498 RCC has no HIF-1α and pVHL can regulate HIF-2α in the similar manner (Maxwell et al., 1999; Ohh et al., 2000; Staller et al., 2003), we detected HIF-2α instead of HIF-1α. The viral infection of Nur77 could not further stabilized HIF-2α in A498/pBabe. On the contrary, Nur77 induced the HIF-2α stabilization in A498/VHL (Figure 6E). Taken together, we suggest that Nur77 indirectly mediated the HIF-α stabilization through binding to pVHL.
Discussion
Initially, Nur77 was identified as an immediate-early gene that is responsive to various growth factors related to cell proliferation (Hazel et al., 1988; Williams and Lau, 1993; Yoon and Lau, 1994; Kolluri et al., 2003; Kim et al., 2005b). Nur77 was also found to play a critical function in T cell and cancer cell apoptosis (Li et al., 2000; Winoto and Littman, 2002; Lin et al., 2004). Thus, these findings indicated that Nur77 might have roles in both cell proliferation and apoptosis.
Previously, we found that hypoxia could induce Nur77 via the specific activation of HIF-1α (Choi et al., 2004). Yoo et al. (2004) also showed that hypoxia and the ectopic overexpression of HIF-1α led to Nur77 induction. Hypoxia is intimately involved in solid tumor progression and angiogenesis, and HIF-1α is a transcription factor and a key regulator of genes expressed under hypoxic conditions (Semenza, 2001, Wenger, 2002), and therefore, HIF-1α is tightly controlled under normoxic conditions. Moreover, pVHL constitutively binds to the hydroxylated HIF-1α subunit, which leads to the degradation of HIF-1α in an ubiquitin-dependent manner (Safran and Kaelin, 2003).
In this study, we studied the mechanism by which Nur77 stabilizes HIF-1α. We found that Nur77 specifically associates with the α-domain of pVHL, the elongin C binding region. On binding to pVHL, Nur77 blocks the elongin C binding region, thus protecting HIF-1α from pVHL-mediated degradation. The ectopic overexpression of Nur77 stabilized HIF-1α and induced its nuclear accumulation (Figure 1B). Moreover, Nur77 increased the transactivation of HIF (Figure 2D and 5C) and the induction of HIF-downstream genes (Figure 5E). Yoo et al. (2004) also reported that Nur77 stabilizes HIF-1α, and showed that TAD-Nur77 stabilized HIF-1α, and that the overexpression of TAD or full-length Nur77 attenuated mdm2 levels. Although mdm2 primarily regulates p53 ubiquitination, it was also found to degrade HIF-1α through ubiquitination (Ravi et al., 2000). However, it remains obscure as to whether mdm2 downregulates HIF-1α, because HIF-1α was found to directly associate with mdm2, and this association was found to subsequently stabilize p53, implying that mdm2 may not degrade HIF-1α through ubiquitination (Chen et al., 2003). In addition, it was also reported that transient transfection with mdm2 increased HIF-1α expression but did not affect the half-life of HIF-1α protein (Bardos et al., 2004).
On the other hand, in the present study, we found that LBD, and not TAD of Nur77, was sufficient to stabilize and transactivate HIF-1α. Full-length or LBD of Nur77 resulted in the stabilization of HIF-1α. And a gradual increase in Nur77 stabilized HIF-1α and increased the interactions between pVHL and HIF-1α (Figure 4D), but weakened the interactions between pVHL and elongin C (Figure 4C). Like Nur77, glucocorticoid receptor (GR) was reported to enhance the transcriptional activity of HIF-1α. In particular, ligand-bound GR-LBD was found to be required for HIF-1α activation (Kodama et al., 2003). However, the effect of the interaction between GR and pVHL on HIF-1α was not further investigated. In addition, some proteins (heat shock protein 90, and CSN5/Jab1) were reported to stabilize HIF-1α in a pVHL-independent manner (Issacs et al., 2002; Bemis et al., 2004).
In this study, we demonstrate that Nur77 interacts with pVHL and modulate its function, and thus stabilizes HIF-1α by inhibiting pVHL-mediated degradation (Figure 7). This mechanism suggests that Nur77 is a target for anticancer drug development for HIF-mediated tumor progression, and that full-length or LBD of Nur77 may be utilized to induce new blood vessel formation.
Abbreviations
- ARNT:
-
aryl hydrocarbon receptor nuclear translocator
- DBD:
-
DNA-binding domain
- EPO:
-
erythropoietin
- GR:
-
glucocorticoid receptor
- HIF:
-
hypoxia inducible factor
- HRE:
-
HIF responsive element
- LBD:
-
ligand-binding domain
- ODD:
-
oxygendependent degradation
- TAD:
-
transactivation domain
- VHL:
-
von Hippel-Lindau
References
Bardos JI, Chau NM, Ashcroft M . Growth factor-mediated induction of HDM2 positively regulates hypoxia-inducible factor 1alpha expression . Mol Cell Biol 2004 ; 24 : 2905 - 2914
Bemis L, Chan DA, Finkielstein CV, Qi L, Sutphin PD, Chen X, Stenmark K, Giaccia AJ, Zundel W . Distinct aerobic and hypoxic mechanisms of HIF-alpha regulation by CSN5 . Genes Dev 2004 ; 18 : 739 - 744
Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J . HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia . EMBO J 2003 ; 22 : 4082 - 4090
Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A . Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia . J Biol Chem 2001 ; 276 : 9519 - 9525
Chen D, Li M, Luo J, Gu W . Direct interactions between HIF-1 alpha and Mdm2 modulate p53 function . J Biol Chem 2003 ; 278 : 13595 - 13598
Choi JW, Park SC, Kang GH, Liu JO, Youn HD . Nur77 activated by hypoxia-inducible factor-1alpha overproduces proopiomelanocortin in von Hippel-Lindau-mutated renal cell carcinoma . Cancer Res 2004 ; 64 : 35 - 39
Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL . Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells . J Biol Chem 2002 ; 277 : 38205 - 38211
Hazel TG, Nathans, Lau LF . A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily . Proc Natl Acad Sci USA 1988 ; 85 : 8444 - 8448
Hellwig-Burgel T, Rutkowski K, Metzen E, Fandrey J, Jelkmann W . Interleukin-1 beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1 . Blood 1999 ; 94 : 1561 - 1567
Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM . Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway . J Biol Chem 2002 ; 277 : 29936 - 29944
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG . HIF alpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing . Science 2001 ; 292 : 464 - 468
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim AV, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ . Targeting of HIF-alphato the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation . Science 2001 ; 292 : 468 - 472
Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L . IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis . FASEB J 2003 ; 17 : 2115 - 2117
Katagiri Y, Takeda K, Yu ZX, Ferrans VJ, Ozato K, Guroff G . Modulation of retinoid signalling through NGF-induced nuclear export of NGFI-B . Nat Cell Biol 2000 ; 2 : 435 - 440
Kim H, Kim YN, Kim H, Kim CW . Oxidative stress attenuates Fas-mediated apoptosis in Jurkat T cell line through Bfl-1 induction . Oncogene 2005a ; 24 : 1252 - 1261
Kim H, Lee JE, Kim BY, Cho EJ, Kim ST, Youn HD . Menin represses JunD transcriptional activity in protein kinase C theta-mediated Nur77 expression . Exp Mol Med 2005b ; 37 : 466 - 475
Kodama T, Shimizu N, Yoshikawa N, Makino Y, Ouchida R, Okamoto K, Hisada T, Nakamura H, Morimoto C, Tanaka H . Role of the glucocorticoid receptor for regulation of hypoxia-dependentgene expression . J Biol Chem 2003 ; 278 : 33384 - 33391
Kolluri SK, Bruey-Sedano N, Cao X, Lin B, Lin F, Han YH, Dawson MI, Zhang XK . Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells . Mol Cell Biol 2003 ; 23 : 8651 - 8667
Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL . HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression . Mol Cell Biol 2001 ; 21 : 3995 - 4004
Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, Xie Z, Fontana JA, Reed JC, Zhang X . Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3 . Science 2000 ; 289 : 1159 - 1164
Li Y, Lin B, Agadir A, Liu R, Dawson MI, Reed JC, Fontana JA, Bost F, Hobbs PD, Zheng Y, Chen GQ, Shroot B, Mercola D, Zhang XK . Molecular determinants of AHPN (CD437)-induced growth arrest and apoptosis in human lung cancer cell lines . Mol Cell Biol 1998 ; 18 : 4719 - 4731
Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK . Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3 . Cell 2004 ; 116 : 527 - 540
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ . The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis . Nature 1999 ; 399 : 271 - 275
Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG . Ubiquitinylation of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein . Nat Cell Biol 2000 ; 2 : 423 - 427
Page EL, Robitaille GA, Pouyssegur J, Richard DE . Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms . J Biol Chem 2002 ; 277 : 48403 - 48409
Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A . Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha . Genes Dev 2000 ; 14 : 34 - 44
Roe JS, Kim H, Lee SM, Kim ST, Cho EJ, Youn HD . p53 stabilization and transactivation by a von Hippel-Lindau protein . Mol Cell 2006 ; 22 : 395 - 405
Safran M, Kaelin WG . HIF hydroxylation and the mammalianoxygen-sensing pathway . J Clin Invest 2003 ; 111 : 779 - 783
Semenza GL . HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus . Cell 2001 ; 107 : 1 - 3
Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W . Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL . Nature 2003 ; 425 : 307 - 311
Stebbins CE, Kaelin WG, Pavletich NP . Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function . Science 1999 ; 284 : 455 - 461
Suzuki S, Suzuki N, Mirtsos C, Horacek T, Lye E, Noh SK, Bouchard D, Mak TW, Yeh WC . Nur77 as a survival factor in tumor necrosis factor signaling . Proc Natl Acad Sci 2003 ; 100 : 8276 - 8280
Wenger RH . Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression . FASEB J 2002 ; 16 : 1151 - 1162
Williams GT, Lau LF . Activation of the inducible orphan receptor gene nur77 by serum growth factors: dissociation of immediate-early and delayed-early responses . Mol Cell Biol 1993 ; 13 : 6124 - 6136
Wilson AJ, Arango D, Mariadason JM, Heerdt BG, Augenlicht LH . TR3/Nur77 in colon cancer cell apoptosis . Cancer Res 2003 ; 63 : 5401 - 5407
Winoto A, Littman DR . Nuclear hormone receptors in T lymphocytes . Cell 2002 ; 109 : S57 - S66
Yoon JK, Lau LF . Involvement of JunD in transcriptional activation of the orphan receptor gene nur77 by nerve growth factor and membrane depolarization in PC12 cells . Mol Cell Biol 1994 ; 14 : 7731 - 7743
Yoo YG, Yeo MG, Kim DK, Park H, Lee MO . Novel function of orphan nuclear receptor Nur77 in stabilizing hypoxia-inducible factor-1alpha . J Biol Chem 2004 ; 279 : 53365 - 53373
Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL . Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics . Cancer Res 2000 ; 60 : 1541 - 1545
Zhou J, Schmid T, Frank R, Brune B . PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1alpha from pVHL-independent degradation . J Biol Chem 2004 ; 279 : 13506 - 13513
Acknowledgements
This work was supported by research grants from the Research for Pure Basic Science, Korea Research Foundation (2002-KRF-C00070, to H.-D. Y.), and in part by Korea Science and Engineering Foundation through the Center for Functional Analysis for Human Genome (3314-20060070).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Experimental & Molecular Medicine website
Supplementary information
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kim, BY., Kim, H., Cho, EJ. et al. Nur77 upregulates HIF-α by inhibiting pVHL-mediated degradation. Exp Mol Med 40, 71–83 (2008). https://doi.org/10.3858/emm.2008.40.1.71
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2008.40.1.71
Keywords
This article is cited by
-
Prolonged cardiac NR4A2 activation causes dilated cardiomyopathy in mice
Basic Research in Cardiology (2022)
-
Transcriptomic analysis of the mouse retina after acute and chronic normobaric and hypobaric hypoxia
Scientific Reports (2021)
-
The nuclear receptors NUR77, NURR1 and NOR1 in obesity and during fat loss
International Journal of Obesity (2012)
-
Involvement of BH4 domain of bcl-2 in the regulation of HIF-1-mediated VEGF expression in hypoxic tumor cells
Cell Death & Differentiation (2011)