Abstract
Advanced glycation endproducts (AGEs) have been reported to play a role in neointimal formation and increase the rate of in-stent restenosis (ISR) in the diabetic coronary artery disease patients treated with stents, but the potential pathogenic mechanisms of AGEs in vascular smooth muscle cell proliferation remain unclear. We sought to determine the AGEs related pathobiological mechanism of diabetic vasculopathy. Rat aortic smooth muscle cell (RAoSMC) culture was done with different concentrations of AGEs and proliferation was assessed. Immunohistochemistry for receptor of AGEs (RAGE) was performed with human carotid atheroma. Western blotting was performed to assess the activation of MAP kinase system in the cultured RAoSMC. AGEs increased RAoSMC proliferation and were associated with increased phosphorylation of ERK and p38 kinase by time and dose dependent manner. The MAP kinase activity was decreased by RNA interference for RAGE. AGEs stimulation increased reactive oxygen species (ROS) generation in cultured RAoSMC. From this study it is concluded that AGEs played a key role in RAoSMC proliferation via MAP kinase dependent pathways. Activation of vascular smooth muscle cell (VSMC) proliferation by MAP kinase system and increased formation of ROS may be the possible mechanisms of AGEs induced diabetic vasculopathy.
Similar content being viewed by others
Introduction
The factors underlying accelerated atherosclerosis in diabetes extend beyond dyslipidemia, hypertension and obesity. Even after correction of these typical risk factors and rigorous control of hyperglycemia, diabetic patients continue to experience increased atherosclerotic vascular disease so called diabetic vasculopathy (Kannel and McGee, 1979; Uusitupa et al., 1990; DCCT research group, 1993). In the era of drug eluting stents (DES), the rate of ISR and major adverse cardiac event (MACE) after stenting are still higher in diabetic patients, affecting 25% of patients treated with coronary artery stenting (Van Belle et al., 1997). It is assumed to be the result of neointimal formation characterized by migration and proliferation of VSMCs, however, the pathogenic mechanisms leading to the exaggerated restenosis in diabetes are poorly understood (Kornowski et al., 1998). AGEs have been linked to the development of various complications in long standing diabetes (Brownlee et al., 1988). Several studies have demonstrated that AGEs and its receptor/ligand interaction play a role in neointima formation after vascular injury irrespective of diabetes status and these findings suggest a novel target to minimize neointimal hyperplasia (Sakaguchi et al., 2003; Stephenson et al., 2003; Zhou et al., 2003). Activation of RAGE results in both removal of irreversibly glycosylated molecules, and activation of cell function, including secretion of various cytokines, which may subsequently induce the proinflammatory mediators (Vlassara et al., 1994; Bierhaus et al., 1997). However, the role of AGEs in the signaling pathways of VSMC proliferation remains to be elucidated.
We hypothesized that increased VSMC proliferation in diabetic vasculopathy is associated with AGEs and activation of the MAPK system may be one of the pathobiologic mechanism. Furthermore, we also investigated the association between AGEs and formation of intracellular ROS.
Materials and Methods
This investigation was conducted after our institutional review board authorization and also conforms with the principles outlined in the Declaration of Helsinki (Cardiovascular Research 1997;35:2-4) and with the Guide for the Care and Use of Laboratory Animals (NIH Publication 85-23).
Reagents
Collagenase type IA and elastase for cell preparation were obtained from Sigma Chemical Co. (St.Louis, MO). Phospho-ERK, phospho-JNK, and phospho-p38 antibodies were from New England Biolabs (Beverly, MA). DMEM and Dulbecco's FBS were obtained from Gibco Life technologies (Gaithersburg, MD). PVDF transfer membrane and ECL Western blotting detection system were purchased from NEN Life Science Products (Boston, MA) and Amersham (Little Chalfont, England), respectively. Reverse Transcription System was obtained from Promega Co. (Madison, WI).
Tissue preparation and immunochemical analysis in human atheroma specimens
Human carotid endarterectomy specimens (n = 9) were obtained from diabetic patients undergone carotid endarterectomy. Each specimen was fixed with 10% buffered formalin and embedded in paraffin. Immunohistochemistry was performed using polyclonal monospecific antibodies to RAGE (Santa Cruz Biotechnology, Santa Cruz, CA). Peroxidase-conjugated goat anti-rabbit IgG (Sigma Chemical Co.) was used to visualize the sites of primary antibody binding to the antigen.
Isolation and culture of RAoSMC and preparation of AGEs
RAoSMCs were isolated as previously described (Lee et al., 2004). Breifly, the aorta was enzymatically isolated from the thoracic aortas from 6-8 weeks-old Spraque-Dawley rats. The aorta was transferred into a plastic tube containing 5 ml of the enzyme dissociation mixture and was incubated for 2 h at 37℃. The suspension was centrifuged (1,500 rpm for 10 min) and the pellet was resuspended in DMEM with 10% FBS. Cells were cultured over several passages (up to 15). RAoSMC were cultured in DMEM supplemented with 10% FCS, 100 IU/ml penicillin, 100 µg/ml streptomysin in 75-cm2 flasks at 37℃ in a humidified atmosphere of 90% air and 10% CO2 (Forma Scientific, Marietta, OH).
RAoSMCs culture was done with different concentrations of AGEs stimulation (1.0, 10, 100, 1,000 µg/ml). AGE-BSA was prepared by incubating BSA (WAKO, Tokyo, Japan) in PBS with 0.7 M glucose for 6 mo at 37℃.
Measurement of cell proliferation
Cell proliferation was measured by PreMix WST-1 Cell Proliferation Assay System (Takara Biomedicals, Tokyo, Japan). This system enables the measurement of cell proliferation with colorimetric assay, and bases on the cleavage of slightly red tetrazolium salt (WST-1) by mitochondrial succinate-tetrazolium reductase in viable cells. As the increase in enzymes activity leads to an increase of the production of formazan dye, the quantity of formazan dye is related directly to the number of metabolically active cells in the medium. Cells (5-7 × 103) were seeded into wells of a 96-well culture plate and incubated with AGE for the times indicated. WST-1 cell proliferation reagent was added directly to the supernatant (10 µl/ 100 µl growth medium), and incubated at 37℃ for 3h. The absorbancy of the solubilized dark red formazan product was then determined at 450 nm.
Immunoblot analysis
Confluent RAoSMCs were cultured for 48 h in serum-free DMEM and were pre-treated with AGE (0~100 µg/ml) for 1 d at 37℃. After treatment with different concentration of AGE, cells were rinsed in ice-cold PBS and treated with lysis buffer (1% Triton X-100, 0.1% mercaptoethanol, 1 mM EDTA, 1 mM EGTA, 50 mM Tris-HCl (pH 7.0), 1 mM PMSF) for 20 min on ice. Cell lysates were collected into microcentrifuge tubes, vortexed, and centrifuged at 12,000 rpm for 20 min. Protein concentration was measured in the supernatant using a DC protein assay reagent according to the manufacturer's instructions and equalized for all samples. Reduced samples (30 g) were subjected to SDS.PAGE (NuPAGE 4-12% Bis-Tris gel) and then electrotransferred to nitrocellulose membrane. For detection of phosphorylated ERK-1/2, membranes were incubated with antibody directed against a phospho-specific ERK-1/2 followed by incubation with goat anti-rabbit IgG conjugated to HRP. ECL detection method was employed for color development.
RNA interference
For function-blocking experiments, we used small interfering RNA molecules (siRNA) targeted at RAGE mRNA. A 21-nt sequence for siRNA was derived from the rat RAGE (GenBank accession no. GI: 81722) obtained from Ambion, Inc. (TX): small interfering RNA against RAGE (sense, 5'-GCUAGAAUGGAAACUGAACTT-3'; antisense, 5'-GUUCAGUUUCCAUUCUAGCTT-3'). Smooth muscle cells were transfected with si-RAGE duplexes by using siPORT NeoFX (Ambion, TX). Briefly, RNA duplex (10 nM of final concentration) was incubated in serum-free DMEM containing 15 µl of siPORT NeoFX for 10 min. The complex was added to the empty 60 mm culture plate and then overlay smooth muscle cell suspension (1 × 105 cells per plate) onto the culture plate wells containing transfection complexes and the transfected cells were incubated in normal cell culture conditions until ready for assay.
Measurement of intracellular ROS generation
RAoSMCs were labeled with 2', 7'-dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular Probe, Eugene, OR) (Lee et al., 2004). The probe H2DCFDA (5M) enters the cell and the acetate group on H2DCFDA is cleaved by cellular esterases, trapping the nonfluorescent 2', 7'-dichlorofluorescin (DCF) inside. Subsequent oxidation by reactive oxygen species yields the fluorescent product DCF. The dye, when exposed to an excitation wavelength of 480 nm, emits light at 535 nm only when it has been oxidized. Labeled RAoSMCs were examined using a luminescence spectrophotometer for oxidized dye. The quiescent cells were treated with AGE for 3 h before labeling with H2DCFDA.
Data analysis
At least three independent experiments were conducted for the analysis. The non-parametric Mann-Whitney U test was used to analyze mean values, and P value of <0.05 was considered statistically significant.
Results
Expression of RAGE in human carotid atheroma
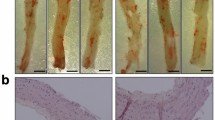
Carotid atheroma specimens showed a thickened intima associated to an area of necrotic core and lipid-laden atheroma in all cases. RAGE was stained as dark brown within atheromatous plaques. RAGE stained cells were distributed mainly in the base of atherosclerotic plaque, in the medio-intimal junction area and its immunoreactivity was colocalized with macrophage, mononuclear cells, and smooth muscle cells (Figure 1).
Immunohistochemistry of RAGE in human carotid atheroma. RAGE was stained as dark brown within atheromatous plaques. RAGE stained cells were distributed mainly in the base of atherosclerotic plaque, in the medio-intimal junction area and its immunoreactivity was co-localized with macrophage, mononuclear cells and smooth muscle cells. Arrow indicates RAGE stained smooth muscle cells.
Proliferative effect of AGEs in cultured RAoSMC
Compared with controls AGEs stimulation group showed increased smooth muscle proliferation. RAoSMC proliferation was increased until AGEs concentration 100 µg/ml suggesting AGEs increase cell proliferation by concentration dependent manner (Figure 2).
Effects of AGEs on phosphorylation of MAPKs in RAoSMC
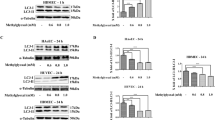
Western blot analysis of p-ERK, p-JNK, p-P38 were performed in cultured RAoSMC with or without AGE stimulation. Compared with control group, VSMC cultured with 50 µg/ml concentration AGE stimulation revealed increased phosphorylation of MAPK system. Activity of p-ERK and p-P38 was markedly increased in AGE stimulated group comparing with p-JNK (Figure 3).
Effects of AGEs on phosphorylation of MAPKs in RAoSMC. Western blot analysis of p-ERK, p-JNK, p-P38 were performed in cultured RAoSMC with or without AGE stimulation. Compared with control group, VSMC cultured with 50 µg/ml concentration AGE stimulation revealed increased phosphorylation of MAPK system. Activity of p-ERK and p-P38 was markedly increased in AGE stimulated group comparing with p-JNK.
Effects of AGEs on phosphorylation of ERK in cultured RAoSMC
Activity of p-ERK was increased in AGEs stimulation group compared with control group. AGEs stimulates phosphorylation of ERK in concentration dependent manner (Figure 4A). Phosphorylation of ERK was increased with the prolonged exposure with the AGEs (Figure 4B).
Effects of AGEs on phosphorylation of ERK in cultured RAoSMC. (A) Effects of different concentration of AGEs stimulation on phosphorylation of ERK. Activity of p-ERK was increased in AGEs stimulation group compared with control group. AGEs stimulates phosphorylation of ERK in concentration dependent manner. (B) Effect of increased AGEs stimulation duration on phosphorylation of ERK. ERK phosphorylation was increased after 30 min of stimulation and continuously increased with the prolonged exposure of AGEs.
Inhibition of RAGE expression by siRNA for RAGE
To further confirm whether the phosphorylation of ERKs was specific response for AGEs treatment, siRNA for RAGE was treated and activation level of ERKs was estimated. Expression of RAGE protein showed reduction of 90% by RNA interference and was detected by Western blot analysis. The ERKs activation increased by AGEs was decreased by RNA interference for RAGE, indicating that AGEs activated ERKs via RAGE (Figure 5).
Inhibition of RAGE expression by siRNA for RAGE. RAoSMCs were transfected with siRNA to a final concentration of 10 nM or vehicle and stimulated by AGE for 3 h. Each signal was detected by immunoblot analysis using anti-ERK antibody and was quantified by densitometrical analysis. Expression of RAGE protein was reduced by RNA interference by Western blot analysis. The ERKs activation increased by AGEs was decreased by RNA interference for RAGE, indicating that AGEs activated ERKs via RAGE.
Effects of AGEs on the formation of intracellular ROS
Confocal microscopy of intracellular DCF revealed increased DCF fluorescence with AGE stimulation concentration dependently suggesting AGEs increased intracellular ROS (Figure 6).
Discussion
One of the challenges in cardiovascular medicine is reducing the complications in patients after percutaneous coronary intervention (PCI), especially for those who with diabetes mellitus, who are particularly prone to ISR. Although ISR have much decreased with the use of DES recently, but DES itself has other unexpected complications such as late stent thrombosis. It is well known that restenosis and overall consequent adverse cardiac events are more frequent in diabetics compared with non-diabetics who underwent PCI even with the DES. This finding may reflect differences in the nature of restenosis in this population compared with that in non-diabetic subjects undergoing comparable interventions. Although, a few studies have identified the clinical and angiographic predictors of restenosis in diabetes patients, all the factors relating to the probability of restenosis after stent deployment in this high-risk patients subgroup are not known (West et al., 2004). Our previous study demonstrated that the rate of angiographic ISR was significantly higher in diabetic patients whose serum AGEs level was high compared with low AGEs group (Choi et al., 2005). In the current study we also demonstrated basal immunoreactivities of RAGE in human atheroma taken from diabetic patients. RAGE stained cells were distributed mainly in the base of the plaque, in the mediointimal junction area and its immunoreactivity was co-localized with smooth muscle cells which advocate the relation between AGE/RAGE interaction and VSMC proliferation.
In the setting of hyperglycemia in diabetes, long-term exposure of free amino groups on polypeptides or lipids to higher levels of glucose eventuates in the formation of AGEs (Brownlee 1995). AGEs are increased at sites of atherosclerotic lesions, especially in diabetes (King and Brownlee, 1996; Chappey et al., 1997). Increased expression of AGEs has also been found in settings like renal failure and amyloidosis, indicating the biology of AGEs extends beyond diabetes. The cellular effects of AGEs are largely mediated by their specific engagement of cell surface receptor RAGE. Studies have demonstrated RAGE expression at a very low level in a range of cells, including endothelial and smooth muscle cells and mononuclear cells, RAGE expression increases and receptor upregulation is sustained when particular pathological processes intervene (Brett et al., 1993; Ritthaler et al., 1995).
Several recent experimental studies showed that AGEs can actively participate in neointimal formation after arterial injuries. Zhou et al. demonstrated that there was significantly increased accumulation of AGEs and increased immunoreactivities of RAGE and S100/calgranulins in carotid artery of diabetic rats in response to balloon injury compared with that of nondiabetic rats. Blockade of the RAGE/ligand interaction significantly decreased the S100-stimulated VSMC proliferation in vitro and suppressed neointimal formation, and increased luminal area in both Zucker diabetic and nondiabetic rats. These findings indicate that RAGE/ligand interaction plays a key role in neointimal formation after PCI, especially in diabetics and suggest the plausibility of RAGE blockade as a therapeutic target in vascular injury, both in euglycemia and diabetes.
An increasing body of literature has begun to elucidate the pathobiological mechanisms underlying the RAGE/ligand interaction. AGEs has been shown to induce significant dose-dependent SMC migration (Higashi et al., 1997), and RAGE/ligand interaction upregulates the production of various cytokines and growth factors such as TNF-α (Miyata et al., 1996) and PDGF (Yamamoto et al., 1996). SMC migration by AGEs was significantly inhibited by an antibody TGF-β, and TGF-β secreted into the culture medium from AGE-stimulated VSMC was 7-fold higher than that of control, suggesting a potential role for RAGE/ligand interaction in TGF-β release after vascular injury (Higashi et al., 1997). In addition, binding of RAGE to its ligand leads to activation of key cell signaling pathways, such as p44/p42 (ERK1/2), p21ras, MAP kinases, NF-κB, cdc42/rac, and JAK/Stat, thereby reprogramming cellular properties (Lander et al., 1997; Huttunen et al., 1999). Adhesion and migration of aortic VSMCs are suppressed by inhibitors of PI3K, ERK, protein tyrosine kinase, and Src kinase (Lee et al., 2006). Angiotensin II induced VSMC proliferation is also associated with increased phosphorylation of ERK 1/2, JNK 1/2 and p-38 (Won et al., 2006).
Balloon injury activates the MAPK pathway in diabetics, and hyperinsulinemia activation of the MAPK pathway has been shown to be of importance in the exaggerated neointimal hyperplasia after balloon injury in diabetic animals (Indolfi et al., 2001). Blockade of RAGE/ligand interaction decreases MAPK activity in cultured SMCs in a concentration-dependent manner (Lander et al., 1997). In our study, we demonstrated VSMC proliferation by AGEs stimulation and these increased VSMC proliferation was due to the increased phosphorylation of ERK and p-38, which are very important in MAPK signaling pathway. AGEs stimulated VSMC proliferation and MAPKs phosphorylation were increased by time and concentration dependent manner. In our data, AGEs induced ERK phosphorylation was continuously increased by the stimulation duration. However, Lander et al. previously demonstrated that AGE induced MAPK activation was peaked at 10 min after stimulation and reduced thereafter. As activation of MAPK is typically an early event in cellular activation, continuous increment of ERK phosphorylation might be caused by secondary activation of other cytokines. This observation should be confirmed by future experiment using ERK downstream molecule. ERK-p-38 signaling is also activated by AGE/RAGE interaction and RAGE blockade reduces its activation (Li et al., 2004). Our data also demonstrated that AGEs stimulation increased p-38 phosphorylation.
Our previous serologic study of diabetic PCI patients revealed high serum levels of AGEs which was independent risk factor for ISR were significantly correlated with HbA1C and time duration of diabetes (Choi et al., 2005). Considering the in vitro and serologic study results, it can be explained that restenosis and increased atherosclerosis of the diabetic patients are related to the high serum levels of AGEs, which represented time dependent exposure of poor glycemic control.
Recent study revealed that AGEs and RAGE interaction triggers the intracellular reactive oxygen species (ROS). Activated NADPH oxidase is a central target of RAGE and that ROS generated by this mechanism may significantly impact on cellular properties (Wautier et al., 2001). Li et al. (2007) recently reported that high extracellular glucose significantly increases ROS generation and AGEs formation in human cardiac myocyte and MAPK activation is one of possible mechanisms of triggering ROS generation. Our study also revealed concentration dependent increment of intracellular ROS by AGEs stimulation. Future work is warranted to examine the relationship between AGEs and ROS generation using such as RAGE RNA interference.
In conclusion, our data suggest a central role for AGEs as a key factor promoting VSMC proliferation and neointimal hyperplasia after stent deployment in diabetic patients. These observations will give a way to the potentially novel target for limiting the development and progression of neointimal hyperplasia by AGEs.
Abbreviations
- AGEs:
-
advanced glycation endproducts
- DES:
-
drug-eluting stents
- ISR:
-
in-stent restenosis
- MACE:
-
major adverse cardiac event
- PCI:
-
percutaneous coronary intervention
- RAGE:
-
receptor of AGEs
- RAoSMC:
-
rat aortic smooth muscle cell
- ROS:
-
reactive oxygen species
- VSMC:
-
vascular smooth muscle cell
References
Bierhaus A, Chevion S, Chevion M, Hofmann M, Quehenberger P, Illmer T, Luther T, Berentshtein E, Tritschler H, Müller M, Wahl P, Ziegler R, Nawroth PP . Advanced glycation endproduct-induced activation of NF-κB is suppressed by alpha-lipoic acid in cultured endothelial cells . Diabetes 1997 ; 46 : 1481 - 1490
Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, Nowygrod R, Neeper M, Przysiecki C, Shaw A . Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues . Am J Pathol 1993 ; 143 : 1699 - 1712
Brownlee M, Cerami A, Vlassara H . AGEs in tissue and the biochemical basis of diabetic complications . N Engl J Med 1988 ; 318 : 1315 - 1321
Brownlee M . Advanced glycosylation in diabetes and aging . Ann Rev Med 1995 ; 46 : 223 - 234
Chappey O, Dosquet C, Wautier MP, Wautier JL . Advanced glycation end products, oxidant stress and vascular lesions . Eur J Clin Invest 1997 ; 27 : 97 - 108
Choi EY, Kwon HM, Ahn C, Lee GT, Joung B, Hong BK, Yoon YW, Kim D, Byun KH, Kang TS, Yoon SJ, Kwon SW, Lee SJ, Park JK, Kim HS . Serum levels of advanced glycation endproducts are associated with in-stent restenosis in diabetic patients . Yonsei Med J 2005 ; 46 : 78 - 85
Diabetes Control and Complications Trial (DCCT) Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus . N Engl J Med 1993 ; 329 : 977 - 986
Higashi T, Sano H, Saishoji T, Ikeda K, Jinnouchi Y, Kanzaki T, Morisaki N, Rauvala H, Shichiri M, Horiuchi S . The receptor for advanced glycation end products mediates the chemotaxis of rabbit smooth muscle cells . Diabetes 1997 ; 46 : 463 - 472
Huttunen HL, Fages C, Rauvala H . Receptor for advanced glycation endproducts (RAGE)-mediated neurite outgrowth and activation of NF-κB require the cytoplasmic domain of the receptor of but different downstream signaling pathways . J Biol Chem 1999 ; 274 : 19919 - 19924
Hwang KC, Lee KH, Jang Y . Inhibition of MEK1,2/ERK mitogenic pathway by estrogen with antiproliferative properties in rat aortic smooth muscle cells . J Steroid Biochem Mol Biol 2002 ; 80 : 85 - 90
Indolfi C, Torella D, Cavuto L, Davalli AM, Coppola C, Esposito G, Carriero MV, Rapacciuolo A, Di Lorenzo E, Stabile E, Perrino C, Chieffo A, Pardo F, Chiariello M . Effects of balloon injury on neointimal hyperplasia in streptozocin-induced diabetes and in hyperinsulinemic nondiabetic pancreatic islet transplanted rats . Circulation 2001 ; 103 : 2980 - 2986
Kannel WB, McGee DL . Diabetes and cardiovascular disease: The Framingham Study . J Am Med Assoc 1979 ; 241 : 2035 - 2038
King G, Brownlee M . The cellular and molecular mechanisms of diabetic complications . Endocrinol Metab Clin North Am 1996 ; 25 : 255 - 270
Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB . In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia . J Am Coll Cardiol 1998 ; 31 : 224 - 230
Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM . Activation of the receptor for advanced glycation endproducts triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress . J Biol Chem 1997 ; 272 : 17810 - 17814
Lee BH, Bae JS, Park RW, Kim JE, Park JY, Kim IS . Big-h3 triggers signaling pathways mediating adhesion and migration of vascular smooth muscle cells through αvβ5 integrin . Exp Mol Med 2006 ; 38 : 153 - 161
Lee KH, Lim S, Kang SM, Kim DH, Cho HK, Chung JH, Kwon HM, Chung H, Lee H, Jang Y, Hwang KC . Antiproliferative mechanisms of raxofelast (IRFI-016) in H2O2-stimulated rat aortic smooth muscle cells . Eur J Pharmacol 2004 ; 484 : 119 - 125
Li JH, Huang XR, Zhu H, Oldfield M, Cooper M, Truong LD, Johnson RJ, Lan HY . Advanced glycation end products activate Smad signaling via TGF-β-dependent and -independent mechanisms: implications for diabetic renal and vascular disease . FASEB J 2004 ; 18 : 176 - 178
Li SY, Sigmon VK, Babcock SA, Ren J . Advanced glycation endproduct induced ROS accumulation, apoptosis, MAP kinase activation and nuclear O-GlcNAcylation in human cardiac myocytes . Life Sci 2007 ; 80 : 1051 - 1056
Miyata T, Hori O, Zhang J, Yan SD, Ferran L, Iida Y, Schmidt AM . The receptor for advanced glycation end products (RAGE) is a central mediator of the interaction of AGR-β2 microglobulin with human mononuclear phagocytes via an oxidant-sensitive pathway: implications for the pathogenesis of dialysis-related amyloidosis . J Clin Invest 1996 ; 98 : 1088 - 1094
Ritthaler U, Deng Y, Zhang Y, Greten J, Abel M, Sido B, Allenberg J, Otto G, Roth H, Bierhaus A, Ziegler R, Schmidt AM, Waldherr R, Wahl P, Stern DM, Nawroth PP . Expression of RAGE in peripheral occlusive vascular disease . Am J Pathol 1995 ; 146 : 688 - 694
Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, Andrassy M, Marso SP, Duda S, Arnold B, Liliensiek B, Nawroth PP, Stern DM, Schmidt AM, Naka Y . Central role of RAGE-dependent neointimal expansion in arterial restenosis . J Clin Invest 2003 ; 111 : 959 - 972
Stephenson K, Tunstead J, Tsai A, Gordon R, Henderson S, Dansky HM . Neointimal formation after endovascular arterial injury is markedly attenuated in db/db mice . Arterioscler Thromb Vasc Bio 2003 ; 23 : 2027 - 2033
Uusitupa MI, Niskanen LK, Siitonen O, Voutilainen E, Pyorala K . Five-year incidence of atherosclerotic vascular disease in relation to general risk factors, insulin level, and abnormalities in lipoprotein composition in non-insulin-dependent diabetic and non-diabetic subjects . Circulation 1990 ; 82 : 27 - 36
Van Belle E, Bauters C, Hubert E, Bodart JC, Abolmaali K, Meurice T, McFadden EP, Lablanche JM, Bertrand ME . Restenosis rates in diabetic patients: a comparison of coronary stenting and balloon angioplasty in native coronary arteries . Circulation 1997 ; 96 : 1454 - 1460
Vlassara H, Bucala R, Striker LJ . Pathogenic effects of advanced glycation: Biochemical, biological, and clinical implications for diabetes and aging . Lab Invest 1994 ; 70 : 138 - 151
Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL . Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE . Am J Physiol Endocrinol Metab 2001 ; 280 : E685 - E694
West NE, Ruygrok PN, Disco CM, Webster MW, Lindeboom WK, O'Neill WW, Mercado NF, Serruys PW . Clinical and angiographic predictors of restenosis after stent deployment in diabetic patients . Circulation 2004 ; 109 : 867 - 873
Won SM, Park YH, Kim HJ, Park KM, Lee WJ . Catechins inhibits angiotensin II-induced vascular smooth muscle cell proliferation via mitogen-associated protein kinase pathway . Exp Mol Med 2006 ; 38 : 525 - 534
Yamamoto Y, Yamagishi S, Hsu CC, Yamamoto H . Advanced glycation end products-receptor interactions stimulate the growth of human pancreatic cancer cells through the induction of platelet-derived growth factor-B . Biochem Biophys Res Commun 1996 ; 222 : 700 - 705
Zhou Z, Wang K, Penn MS, Marso SP, Lauer MA, Forudi F, Zhou X, Qu W, Lu Y, Stern DM, Schmidt AM, Lincoff AM, Topol EJ . Receptor for AGE (RAGE) mediates neointimal formation in response to arterial injury . Circulation 2003 ; 107 : 2238 - 2243
Acknowledgements
This work is supported by research grant of Korean Society of Circulation and BK21 Project for Medical Science, Yonsei University and Korean Instute of Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yoon, Y., Kang, T., Lee, B. et al. Pathobiological role of advanced glycation endproducts via mitogen-activated protein kinase dependent pathway in the diabetic vasculopathy. Exp Mol Med 40, 398–406 (2008). https://doi.org/10.3858/emm.2008.40.4.398
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2008.40.4.398
Keywords
This article is cited by
-
Hyperglycaemia cause vascular inflammation through advanced glycation end products/early growth response-1 axis in gestational diabetes mellitus
Molecular and Cellular Biochemistry (2019)
-
Effects of glucagon-like peptide-1 on advanced glycation endproduct-induced aortic endothelial dysfunction in streptozotocin-induced diabetic rats: possible roles of Rho kinase- and AMP kinase-mediated nuclear factor κB signaling pathways
Endocrine (2016)
-
A bis-Schiff base of isatin improves methylglyoxal mediated insulin resistance in skeletal muscle cells
Archives of Pharmacal Research (2015)
-
KCa3.1 channels mediate the increase of cell migration and proliferation by advanced glycation endproducts in cultured rat vascular smooth muscle cells
Laboratory Investigation (2013)
-
RhoA/ROCK-dependent moesin phosphorylation regulates AGE-induced endothelial cellular response
Cardiovascular Diabetology (2012)