Abstract
Coffin–Siris Syndrome (CSS, MIM 135900) is a rare genetic disorder, and mutations in ARID1B were recently shown to cause CSS. In this study, we report a novel ARID1B mutation identified by whole-exome sequencing in a patient with clinical features of CSS. We identified a novel heterozygous frameshift mutation c.1584delG in exon 2 of ARID1B (NM_020732.3) predicting a premature stop codon p.(Leu528Phefs*65). Sanger sequencing confirmed the c.1584delG mutation as a de novo in the proband and that it was not present either in her parents, half-sister or half-brother. Clinically, the patient presented with extreme obesity, macrocephaly, hepatomegaly, hyperinsulinism and polycystic ovarian syndrome (PCOS), which have previously not been described in CSS patients. We suggest that obesity, macrocephaly, hepatomegaly and/or PCOS may be added to the list of clinical features of ARID1B mutations, but further clinical reports are required to make a definite conclusion.
Similar content being viewed by others
Introduction
Coffin–Siris Syndrome (CSS, MIM 135900) is a rare genetic disorder. Proposed minimal criteria for the clinical diagnosis of CSS are developmental or cognitive delay, which may range from mild to severe, distinctive facial features that coarsen over time, hypertrichosis and hypoplastic or absent fifth distal phalanx or nail.1, 2 Also, other ectodermal, constitutional and/or organ-related features may be present.3 CSS is now classified as a BAF (also known as SWI/SNF) complex disorder,4 as several syndrome-related genes that encode subunits of the BAF complex — ARID1A, ARID1B, SMARCA4, SMARCB1, SMARCA2 and SMARCE1 — have been identified.5, 6, 7 The BAF complex modulates chromatin structure and has important roles in transcription, cell differentiation, DNA repair and tumor suppression as reviewed by Hargreaves and Crabtree.8 Heterozygous mutations of these genes are inherited in an autosomal dominant manner, but they usually result from a de novo mutation. They are present in at least half of the patients with CSS.4, 7, 9 Recently, Wieczorek et al7 added another causative factor for CSS — the PHF6 gene.
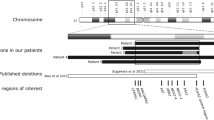
Now, at least 87 patients with mutation, deletion, duplication or translocation affecting ARID1B (or BAF250B) have been described.4, 5, 6, 7, 9, 10, 11, 12, 13, 14 Most of them had a clinical diagnosis of CSS, but it can be due to careful clinical preselection for molecular study.4, 5, 6, 7, 9 In some patients, the mutation in ARID1B was found when searching the cause of unexplained intellectual disability (ID) without CSS diagnosis.11, 12
In this study, we report a novel ARID1B mutation identified by whole-exome sequencing in a patient with clinical features characteristic to CSS.
Case report
The patient is a 16-year-old female. She is the only child of a non-consanguineous Estonian couple and was born from stimulated vaginal delivery as postterm with birth weight 3190 g (−1 SD), length 50 cm (−1 SD) and head circumference (HC) 36 cm (+0.5 SD). Apgar scores were 7/7, and she needed intensive care after birth.
At the age of 14 months, the patient was suspected to have autistic behavior. Also, delayed motor development was noticed — she started to walk independently at 18 months. At that time, her brain MRI showed changes characteristic to perinatal hypoxic-ischemic encephalopathy.
At the age of 2.5 years, increased HC was noticed; her HC was 51 cm (+2 SD). She had overall coarse facial features with long eyelashes and broad nasal bridge (Figure 1a), hepatomegaly (sagittal view 8.9 cm), autistic behavior and absent speech. Chromosomal analysis revealed a normal female karyotype (46, XX). Basic metabolic analyses were normal. Mucopolysaccharidosis was suspected based on radiographic findings as well as clinical features, but the urinary glycosaminoglycans analysis was normal.
Facial view of the patient (a) at the age of 2.5 years, note coarse facial features with long eyelashes and broad nasal bridge; (b) at the age of 6.5 years, note progressively coarsened facial features; (c) at the age of 16 years. (d) brachydactyly, short F5; (e) X-ray of both hands showed hypoplastic distal phalanges.
At the age of 6.5 years, her facial features were progressively coarsened (Figure 1b), she also had excessive body hair and obesity (weight 31 kg, height 120 cm; body mass index (BMI) 21.5 kg/m2, greater than +2 SD). Repeated brain MRI showed for Arnold–Chiari malformation type I.
At the age of 9 years, her weight was 48 kg (+3 SD), height 132 cm (−1.5 SD), BMI=27.6 kg/m2 (greater than +2 SD) and macrocephaly was noticed (HC 58 cm, +4 SD). According to the mother, the patient started to gain weight 2 years earlier when she was on risperidone for 2 months. She was motorically clumsy with normal muscle tonus and answered to simple questions. Phenotypically, she demonstrated acanthosis nigricans on her neck, armpits and inguinal areas, a wide round face, long sparse and loose hair, hypertrichosis, dysmorphic ears, brachydactyly, short F5 (Figure 1d), syndactyly of T2-3 and dysmorphic nails of the F5 and T5. She was tested to have a mild-to-moderate ID (IQ 44). X-ray of both hands showed hypoplastic distal phalanges (Figure 1e). At this time, the diagnosis of CSS was first considered.
At the age of 13 years, her weight was 93.5 kg (+5 SD), height 156.5 cm (−1.5 SD), HC 62.5 cm (+5 SD). BMI was 40 kg/m2 (greater than +3 SD). Phenotype remained unchanged, and hepatomegaly (12.5 cm, +2 SD) persisted. Biochemical analyses showed hyperinsulinaemia (fasting insulin level 42.1 mU/l) and treatment with metformin was started. Electrocardiogram showed a repolarization defect, and echocardiography revealed mild aortic valve insufficiency.
At the age of 16 years, her weight was 109 kg (+7 SD), height 158.7 cm (−2 SD), HC 62.5 cm (+5 SD). BMI was 43 kg/m2 (>+3 SD). She formally fulfilled the Rotterdam criteria for diagnosing polycystic ovarian syndrome (PCOS).15 She had oligoovulation/anovulation (persistent amenorrhea after single episode of uterine bleeding at the age of 13.6 years) and signs of hyperandrogenemia (hypertrichosis, acne beyond the typical age, mildly elevated DHEAS level). The levels of 17-hydroxyprogesterone and gonadotropins were normal. There were no typical findings of PCOS on the pelvic ultrasound; however, the signs of uterine hypoestrogenisation were evident (cervix to corpus ratio only 1:1, thin endometrium). She exhibited a low masculine voice, developed signs of steatohepatitis (mild elevation of aminotransferases), mild changes in cholesterol profile (typical for metabolic syndrome) and strong intensifications of acanthosis nigricans. Other clinical features remained unchanged (Figure 1c).
Over the years, atypical Prader–Willi and Beckwith–Wiedemann syndromes were also considered, but excluded by specific molecular tests. Chromosomal microarray analysis was normal.
Results
We performed whole-exome sequencing of a parent–offspring trio on genomic DNA extracted from blood samples. Exome-sequencing method and data filtering steps have been described previously.16 Mutation analysis was performed by Sanger sequencing in the proband, his parents and two half-sibs.
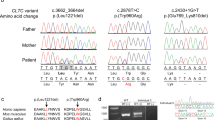
The average exome-sequence coverage per nucleotide per individual was 100 × . For variant analysis and evaluation, we applied SimulConsult’s (SimulConsult, Chestnut Hill, MA, USA) diagnostic decision support software. After variant prioritization, we identified a novel heterozygous frameshift mutation c.1584delG in exon 2 of ARID1B (NM_020732.3) predicting a premature stop codon p.(Leu528Phefs*65). Sanger sequencing confirmed the c.1584delG mutation as a de novo in the proband and not present either in her parents, half-sister or half-brother (Figure 2). The ARID1B variant c.1584delG identified in this study was submitted to the Leiden Open Variation Database (LOVD) at www.lovd.nl/ARID1B. Other selected variants and their exclusion criteria are given in Supplementary Table 1.
Discussion
We describe here a patient with a novel mutation in one of the components of the BAF complex—the ARID1B gene. Our patient fulfils all the minimal criteria needed for the clinical diagnosis of CSS. However, unlike other patients described, she has extreme macrocephaly, obesity with hepatomegaly, PCOS, hyperinsulinism and metabolic syndrome.
BAF is one of the many chromatin-remodeling complexes present in human cells.17 The role of BAF complex in human disorders was recently reviewed by Hargreaves and Crabtree.8 So far, only nonsense and frameshift mutations in ARID1B as well as whole-gene deletions have been identified in patients with CSS.4, 5, 6, 12 In our patient, a 1-bp deletion c.1584delG in ARID1B predicted to cause a frameshift and a premature termination of the protein p.(Leu528Phefs*65) was identified. This alteration most likely results in loss-of-function of the protein, suggesting haploinsufficiency as a disease-causing mechanism.
To compare the clinical characteristics between the patients, we give an overview of all previously described patients with mutation in the ARID1B gene in addition to our patient (Supplementary Table 2). So far, all described patients with ARID1B changes have HC within normal limits or demonstrated microcephaly, except for one patient in Hoyer et al12 and one in Wieczorek et al7 group. Our patient has significant macrocephaly (+5 SD) and Arnold–Chiari malformation type I. Moreover, the macrocephaly in our patient was progressive, which is a very unusual feature and has not been described before. Postnatal weight was described in eight patients and was significantly increased only in our patient (+7 SD). Forty-nine percent were short statured (<2 SD), including our case, but many other described patients had a tendency toward shorter height than expected according to the age, although within normal limits. All patients had developmental delay and/or ID and speech delay ranging from mild to severe. Autistic features were described in 5/7 (71%). Among the patients with ARID1B mutation, the phalangeal changes may or may not exist.4, 9 In our overview, 51/79 (73%) had this finding including our case. Hypertrichosis was described in 94% of patients. None of the other described patients have hyperinsulinism, PCOS or metabolic syndrome. However, because of the high prevalence of PCOS in the general population,18 we should also consider the possibility of coincidence. The second possibility is that the combination of new features of our patient is due to another mutation in some other gene. However, no other disease-causing de novo mutation was identified (Supplementary Table 1).
In conclusion, we suggest that obesity, macrocephaly, hepatomegaly and/or PCOS may be added to the list of clinical features of ARID1B mutations, but further clinical reports are required to make a definite conclusion.
References
Fleck BJ, Pandya A, Vanner L, Kerkering K, Bodurtha J : Coffin-Siris syndrome: review and presentation of new cases from a questionnaire study. Am J Med Genet 2001; 99: 1–7.
Coffin GS, Siris E : Mental retardation with absent fifth fingernail and terminal phalanx. Am J Dis Child 1970; 119: 433–439.
Schrier SA, Bodurtha JN, Burton B et al: The Coffin-Siris syndrome: a proposed diagnostic approach and assessment of 15 overlapping cases. Am J Med Genet A 2012; 158A: 1865–1876.
Tsurusaki Y, Okamoto N, Ohashi H et al: Coffin-Siris syndrome is a SWI/SNF complex disorder. Clin Genet 2013, e-pub ahead of print 1 July 2013; doi:10.1111/cge.12225.
Santen GW, Aten E, Sun Y et al: Mutations in SWI/SNF chromatin remodeling complex gene ARID1B cause Coffin-Siris syndrome. Nat Genet 2012; 44: 379–380.
Tsurusaki Y, Okamoto N, Ohashi H et al: Mutations affecting components of the SWI/SNF complex cause Coffin-Siris syndrome. Nat Genet 2012; 44: 376–378.
Wieczorek D, Bogershausen N, Beleggia F et al: A comprehensive molecular study on Coffin-Siris and Nicolaides-Baraitser syndromes identifies a broad molecular and clinical spectrum converging on altered chromatin remodeling. Hum Mol Genet 2013; 22: 5121–5135.
Hargreaves DC, Crabtree GR : ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res 2011; 21: 396–420.
Santen GW, Aten E, Vulto-van Silfhout AT et al: Coffin-Siris syndrome and the BAF-complex: genotype-phenotype study in 63 patients. Hum mutat 2013; 34: 1519–1528.
Nagamani SC, Erez A, Eng C et al: Interstitial deletion of 6q25.2-q25.3: a novel microdeletion syndrome associated with microcephaly, developmental delay, dysmorphic features and hearing loss. Eur J Hum Genet 2009; 17: 573–581.
Halgren C, Kjaergaard S, Bak M et al: Corpus callosum abnormalities, intellectual disability, speech impairment, and autism in patients with haploinsufficiency of ARID1B. Clin Genet 2012; 82: 248–255.
Hoyer J, Ekici AB, Endele S et al: Haploinsufficiency of ARID1B, a member of the SWI/SNF-a chromatin-remodeling complex, is a frequent cause of intellectual disability. Am J Hum Genet 2012; 90: 565–572.
Michelson M, Ben-Sasson A, Vinkler C et al: Delineation of the interstitial 6q25 microdeletion syndrome: refinement of the critical causative region. Am J Med Genet A 2012; 158A: 1395–1399.
Kosho T, Okamoto N, Ohashi H et al: Clinical correlations of mutations affecting six components of the SWI/SNF complex: detailed description of 21 patients and a review of the literature. Am J Med Genet A 2013; 161: 1221–1237.
Azziz R : Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome: the Rotterdam criteria are premature. J Clin Endocrinol Metab 2006; 91: 781–785.
Vaher U, Noukas M, Nikopensius T et al: De novo SCN8A mutation identified by whole-exome sequencing in a boy with neonatal epileptic encephalopathy, multiple congenital anomalies, and movement disorders. J Child Neurol 2013, e-pub ahead of print 18 December 2013.
Ronan JL, Wu W, Crabtree GR : From neural development to cognition: unexpected roles for chromatin. Nat Rev Genet 2013; 14: 347–359.
Franks S : Polycystic ovary syndrome. N Engl J Med 1995; 333: 853–861.
Acknowledgements
This work was supported by the Estonian Science Foundation (grant no. 8175), targeted Financing from the Estonian Ministry of Education and Research (grant no. SF0180142s08), the EU FP7 (grant no 313010), the Development Fund of the University of Tartu (grant no. SP1GVARENG) and the European Regional Development Fund to the Center of Excellence in Genomics (EXCEGEN; grant no 3.2.0304.11-0312).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Vals, MA., Õiglane-Shlik, E., Nõukas, M. et al. Coffin–Siris Syndrome with obesity, macrocephaly, hepatomegaly and hyperinsulinism caused by a mutation in the ARID1B gene. Eur J Hum Genet 22, 1327–1329 (2014). https://doi.org/10.1038/ejhg.2014.25
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2014.25