Abstract
Neutral lipid storage disease comprises a heterogeneous group of inherited disorders characterized by severe accumulation of cytoplasmic triglyceride droplets in several tissues and neutrophils. A novel type of autosomal recessive lipid myopathy due to PNPLA2 mutations was recently described with associated cardiac disease, myopathy and frequent infections, but without ichthyosis. Here we describe the clinical and biochemical characteristics of a long surviving patient and report on four carrier family members with diverse clinical involvement. Interestingly, heterozygous patients show neutral lipid storage in muscle and in the keratocytes of the skin, Jordans’ bodies, mild myopathy and frequent infections. Biochemical analysis of fibroblasts obtained from patients revealed increased triglyceride storage and reduced lipid droplet-associated triglyceride hydrolase activity. Together, our data implicate that the wild-type allele cannot fully compensate for the mutated dysfunctional allele of PNPLA2 leading to triglyceride accumulation in muscle and mild myopathy in PNPLA2 mutation carriers. The presence of neutral lipid droplets in the skin in PNPLA2 mutation carriers strengthens the link between NLSD and other neutral lipid storage diseases with ichthyosis.
Similar content being viewed by others
Introduction
Neutral lipid storage disease (NLSD) comprises a heterogeneous group of autosomal recessive disorders characterized by severe accumulation of triglyceride (TG) cytoplasmic droplets in several tissues.1, 2 The most common finding in these patients is the presence of May–Grunwald–Giemsa-negative lipid droplets (LDs) (Jordans’ bodies) in otherwise normal neutrophils and eosinophils from peripheral blood.3 Most of the patients with NLSD have lipid storage in skeletal muscle and liver, associated with sever ichthyosis. A distinctive type of autosomal recessive NLSD was defined recently, with prominent myopathy but without ichthyosis. Fischer et al4 described three affected women, from three families of Dutch, French and Algerian origin, who showed marked TG storage in muscle and delays in walking, variable cardiac abnormalities and hepatomegaly. These patients had no mutations in the CGI-58 gene, but they harbored four distinct mutations in the PNPLA2 gene. This form of NLSD, caused by PNPLA2 mutations has been delineated as NLSD with myopathy.4, 5 Several patients have been discovered with NLSD with myopathy in the past few years, and in most cases the different mutations in the PNPLA2 gene resulted in cardiomyopathy and early cardiac lethality in young adulthood (Table 1).4, 5
PNPLA2 catalyzes the initial step in the breakdown of intracellular TGs and, as expected, PNPLA2-defective patients show impaired degradation of cytoplasmic TGs.4 Recent studies have provided new insights into the genetic basis of a group of disorders of lipid metabolism characterized pathologically by TG accumulation in muscle.6, 16, 17, 18, 19, 20, 21, 22 The identification of causative genes offers new tools for diagnosis and genetic counseling. Although novel drugs have shown promising results in vitro, therapy for these disorders remains inadequate. Additional cases are needed to further define the clinical spectrum and genetic heterogeneity of this rare condition. In the current paper, we describe the phenotype and disease course of the Dutch index patient and her family and study the clinical consequences of PNPLA2 heterozygosity.
Subjects and methods
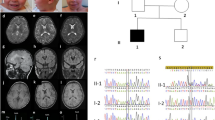
Patient II-3 (described as a child with a compound heterozygous PNPLA2 mutation)4 was born to non-consanguineous, apparently healthy parents (for pedigree, see Figure 1a). She was investigated as a child for myopathy, fatigue and recurrent infections. She had normal intelligence. At the age of 4 years she was diagnosed with a lipid myopathy. Peripheral blood leukocytes showed typical cytoplasmic LDs (Jordans’ bodies; Figures 1b and 2a). Biochemical, histological and electron microscopy investigations of skin and muscle revealed a generalized triglyceridosis with intracellular lipid accumulation. Muscle biopsy demonstrated lipid storage (Figures 2b–d). She had no clinical signs of lipid accumulation in the brain, lungs or heart. She had no ichthyosis, but she did have neutral lipid storage in a skin biopsy. Hormonal investigations were also normal. In young adulthood, she was found to be a compound heterozygote for mutations in the PNPLA2 gene (1-bp deletion, c.808delC, leading to a premature stop codon at amino-acid position 319 (p.Frameshift319*) and a missense mutation c.584C>T, changing proline to leucine (p.P195L). In her 30s, the patient developed hearing loss and progressive proximal muscle weakness. She developed psoriasis and suffered from unexplained, recurrent episodes of short collapses.
Patient I-2 (heterozygous carrier) is the mother of the patient II-3, and she is 61 years old. As a child, she was reported to have frequent infections. She has been diagnosed with a mixed hearing loss and uses a hearing aid since the age of 5 years. She has no symptoms of myopathy or cardiac disease, liver abnormalities or diabetes. She has recurrent episodes of migraine and occasional muscle pain episodes. At neurological investigation, she has no detectable abnormalities.
Patient I-1, (heterozygous carrier) the father of the patient has hearing problems because of recurrent otitis as a child. At this moment, he uses a hearing aid owing to conductive hearing loss. He has no myopathy or fatigue, cardiac disease or diabetes. Since 2008, he has had psoriasis.
Patient III-3 (heterozygous carrier with symptoms) is the son of patient II-3, and he is 7 years old. He has recurrent infections and he is known to have a history of exercise intolerance and concentration problems at school. He cannot fully participate in regular school activities and gymnastics owing to easy fatigue. Neurological examination reveals a decreased muscle tone and winged scapula, no Gowers’ sign of decreased strength and normal reflexes. On regular exercise, his muscle strength improved significantly, but his muscle tone remains somewhat decreased.
Patient II-2, (heterozygous carrier with symptoms) the 36-year-old sister of patient 1 has a mild myopathy. She also has a history of recurrent infections, but no cardiac disease or diabetes. She is reported to have exercise intolerance. Neurological examination reveals a positive Gowers sign and decreased strength and normal reflexes. The patient is married to a third-degree relative on the father’s side, who is not a PNPLA2 mutation carrier. They have two children.
Patient III-1 (heterozygous carrier with symptoms) is the son of patient II-2, and he is 7 years old. He has recurrent infections and he is known with a history of exercise intolerance, confirmed by the ‘six minutes’ walk test. Neurological examination reveals a decreased muscle tone and scapula alatae, but normal reflexes.
Patient III-2 (heterozygous carrier) is the daughter of patient II-2, and she is 4 years old. She has recurrent infections but no obvious exercise intolerance. Neurological examination is normal.
Genetic studies
Sequence analysis of the PNPLA2 gene was performed as described by Fischer et al.4
Laboratory investigations and histology
Histological evaluation and electron microscopy on skin biopsy and muscle were performed according to standard clinical protocols. Light microscopy of frozen transverse sections of skeletal muscle biopsy was stained by Sudan Black B.
Jordans’ bodies were assessed by light microscopy of peripheral blood smear and by electron microscopy of leukocytes after centrifuging citrate blood.
The insulin resistance test was done with a glucose infusion rate 3.3 mg/kg/min, normal range 4–8 mg/kg/min.23
Biochemical studies on TG content of fibroblasts
Fibroblasts were cultivated in Dulbecco’s modified Eagle’s medium containing 10% FCS, 100 IU/ml penicillin and 100 μg/ml streptomycin at 37 °C, 7.5% CO2, 95% humidified atmosphere until confluence and incubated for 24 h in the presence or in the absence of 500 μM oleic acid complexed to BSA at a FFA/BSA ratio of 3:1.16 Thereafter, cells were washed with phosphate-buffered saline (PBS) and total cell lipid was extracted using hexane/isopropanol (3:2). The extracts were dried and resuspended in 1% Triton X-100. TG were determined using the Infinity TG kit (Thermo Electron Corporation, Noble Park, VIC, Australia). Cells were lyzed using 0.1% SDS/0.3 N NaOH and protein content was measured using the BCA reagent (Pierce, Rockford, IL, USA).
Labeling and isolation of LDs
For radioactive labeling of TG stores, confluent cell layer was incubated for 24 h in the presence of 0.5 mM oleic acid (4 mCi 3H-9,10-oleate/mmol) (GE Healthcare, Frederick, MD, USA) complexed to BSA at a FFA/BSA ratio of 3:1.16 For the isolation of LD, cells were washed extensively with PBS and collected using a cell scraper. After centrifugation (200 g, 5 min) the cells were resuspended in buffer A (0.25 M sucrose, 1 mM EDTA, 1 mM dithiothreitol, 20 μg/ml leupetine, 2 μg/ml antipain, 1 μg/ml pepstatin, pH 7.0) and disrupted by sonication (Virsonic 475, Virtis). The lysate was centrifuged to remove cell debris and nuclei at 1000 g and 4 °C for 15 min. The supernatant was transferred to SW41 tubes, overlaid with buffer B (50 mM potassium phosphate (pH 7.4), 100 mM KCl, 1 mM EDTA, 20 μg/ml leupetine, 2 μg/ml antipain, 1 μg/ml pepstatin) and centrifuged in a SW41 rotor (Beckman, Fullerton, CA, USA) for 2 h at 100 000 g and 4 °C. LD were collected as a white band from the top of the tubes and concentrated by centrifugation for 15 min at 20 000 g and 4 °C. The underlying solution was removed and the LD were resuspended by brief sonication in buffer B. TG and protein content of the isolated LD was determined using the Infinity TG kit of the Bradford reagent (Biorad Laboratories GmbH, Munchen, Germany).
Assay for TG hydrolase activity in purified LDs
To determine LD-associated TG hydrolase activity of fibroblasts 150 μl 3H-9,10-oleate radiolabeled LDs in buffer B were mixed with 50 μl fatty acid-free BSA (20%, w/v).16 After incubating the mixture at 37 °C for 1 h, the reaction was terminated by adding 3.25 ml of methanol/chloroform/heptane (10:9:7) and 1 ml of 0.1 M potassium carbonate, 0.1 M boric acid (pH 10.5). After centrifugation (800 g, 15 min), the radioactivity in 1 ml of the upper phase was determined by liquid scintillation counting.
Results
Clinical studies
Echocardiography in patient II-3 (compound heterozygous index patient) demonstrated hypokinesia of the mid- and basal inferior wall of the heart. Magnetic resonance imaging (MRI) of the heart showed diffuse hypokinesia of the left and right ventricles. Lipid accumulation could not be demonstrated using the present software. The ejection fraction was decreased to 43%. Because of suspected cardiac rhythm abnormalities as a cause of the recurrent collapses, a cardiac reveal was implanted for a period of 2 years to register her heart rhythm. Once an episode of ventricular tachycardia was documented, the patient received an implantable cardioverter-defibrillator.
MRI of the legs showed extreme fat deposition in the Soleus muscle (Figure 1c).
MRI and magnetic resonance spectroscopy (MRS) of the liver showed no hepatomegaly nor lipid accumulation, and an asymptomatic liver adenoma was detected.
Insulin sensitivity was measured by a hyperinsulinemic euglycemic clamp (insulin infusion rate 60 mE/m2). Glucose infusion rate at steady state conditions (plasma insulin concentration 56 mU/l) was 18.1 μmol/kg/min, which is substantially decreased compared with an age- and body mass-matched control group (53.9±4.3 μmol/kg/min, see Tack et al23).The findings are consistent with frank insulin resistance.
Patient III-3 (heterozygous carrier with symptoms): MRI and MRS of the brain showed no lipid accumulation.
Audiometric investigations
In patient II-3, audiometry showed a conductive hearing loss with a Fletcher index of 57 dB.
In patient I-2, audiometry showed a mixed hearing loss at 55 dB. In patient I-1, audiometry showed a bilateral conductive hearing loss at 60 dB.
Genetic studies
Patient I-2, the mother of the index patient, was heterozygous for the c.584C>T/p.P195L mutation, whereas the father, patient I-1 and the sister, patient II-2, were heterozygous for c.808delC/p.Frameshift319* mutation.
Heterozygosity for c.584C>T/p.P195L was detected in patient III-3 and the c.808delC/p.Frameshift319* was confirmed in patient III-1 and III-2.
Laboratory investigations
Patient II-3 had an increased creatine kinase (CK 482 U/l normal<170 U/l). Serum lactic acid, pyruvic acid and acyl-carnitine profile levels are within the normal range in all patients. Patient II-2, III-1, III-2 and III-3 had a CK level of 138, 57, 62 and 158 U/l, respectively (normal). None of the family members has hyperglycemia or hyperlipidemia (TGs, total cholesterol levels and subfractions were normal).
Peripheral blood leukocytes show Jordans’ bodies in all investigated family members in the range of 6–12%.
Pathological examinations
Patient II-3 (compound heterozygous index patient): electron microscopy examination and routine blood smear of blood revealed the presence of large neutral LDs in the granulocytes (Figure 2a). Light microscopy of muscle biopsy at age 6 years showed numerous accumulations of cytoplasmic droplets containing neutral lipids (TGs). Transmission electron microscopy of longitudinal sections of skeletal muscle biopsy confirmed the presence of these LDs of up to more than 200 μm width between the sarcomeric filament. In a muscle biopsy taken at the age of 21 years, the LDs were even greater, and were found between the sarcomeric filaments and under the muscle membrane, where they reached a width of more than 1 mm. These LDs were present in all muscle fiber types, but the larger ones were found in the type 1 fibers (Figures 2b–d). The LDs could also be found in a Schwann cell of an intramuscular nerve fiber (not shown). Examination of a skin biopsy at age 3 years demonstrated a small layer of empty vacuoles just under the epithelial basal membrane (Figure 3a), that could already be seen on light microscopy level. On electron microscopy the keratocytes just above the basal membrane contained small neutral LDs (Figure 3b). Some dermal fibroblasts also contained small neutral LDs (not shown).
Patient II-2 (heterozygous carrier sister with symptoms): light microscopy showed mild abnormalities of neutral lipid storage in muscle at age 35 years, especially in type 1 fibers. A few type 1 fibers were somewhat atrophic (Figure 4a). Electron microscopy, however, confirmed an increased subsarcolemmal and cytoplasmic storage of LDs that were smaller than in the index patient (Figure 4b). Light and electron microscopy of skin at age 5 years revealed no vacuoles or LDs (Figures 5a and b). Patient III-3 (heterozygous carrier): electron microscopy of peripheral blood at age 4 years revealed a few small LDs in the granulocytes (Figure 4c)
(a) Muscle biopsy of the heterozygous carrier sister, (II-2) at age 35 years with centrally located small type 1 fiber filled with black-stained LDs. (b) Interfilamentous and subsarcolemmar lipid deposition on EM. (c) Neutral LDs (arrows) in granulocytes of the heterozygous sister (III-3) at age 4 years. ((a) Sudan Black B, (b and c) EM, bar=1 μm).
Biochemical studies
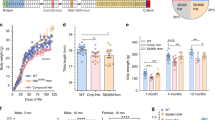
To investigate how heterozygous mutations of PNPLA2 affect TG metabolism in vitro, we determined TG content and TG hydrolase activity in cultured fibroblasts obtained from patients heterozygous for the p.P195L (patient III-3) and the p.Frameshift319* (patient I-2, patient II-2) mutation. In comparison to wild-type fibroblasts, TG content in mutant fibroblasts was significantly increased under standard culture conditions (2.2-, 1.9- and 2-fold for patient III-3, I-2 and II-2, respectively, (Figure 6a) and in the presence of exogenously added fatty acids (2-, 1.6- and 1.7-fold, for patients III-3, I-2 and II-2, respectively, (Figure 6b). Activity assays revealed that the LD-associated TG hydrolase activity of fibroblasts from patients III-3, I-2 and II-2 amounted to 37%, 41% and 56% of normal, respectively (Figure 6c).
TG ontent and LD-associated TG hydrolase activity of wild-type and mutant fibroblasts heterozygous for the p.P195L (III-3) and the p.Frameshift319* (I-2), (II-2) mutation. TG content in fibroblasts was determined under standard culture conditions (a) and in the presence of exogenously added oleic acid (500 μM, b). LD-associated TG hydrolase activity was determined by self-digestion of isolated LDs (c). Data are shown as mean±SD (*P<0.05, **P<0.01, ***P<0.001). In comparison to wild-type fibroblasts, TG content in mutant fibroblasts was significantly increased under standard culture conditions (2.2-, 1.9- and 2-fold, for patient 2, 3 and 5, respectively, a) and in the presence of exogenously added fatty acids (2-, 1.6- and 1.7-fold for patients 2, 3 and 5, respectively, b). Activity assays revealed that the LD-associated TG hydrolase activity of fibroblasts from patients III-3, I-2 and II-2, amounted to 37%, 41% and 56% of normal, respectively (c).
Discussion
Several patients have been recently reported with recessive PNPLA2 mutations and myopathy, but no other organ involvement.5, 11, 12, 14 Here we report on additional unique findings in four carrier family members with diverse clinical involvement, including significant neutral lipid storage in muscle. The clinical features in these individuals included muscle weakness, episodes of muscle pain, sensorineural or conductive deafness and frequent infections in heterozygous individuals, carrying either the p.P195L or the mutation p.Frameshift319* in PNPLA2 (Table 1). The observation of recurrent cardiovascular collapse and other cardiac and endocrine findings in our patient emphasize the need for targeted screening, regular follow-up and preventive treatment in patients with recessive PNPLA2 mutations. It is interesting to note that the compound heterozygous index patient (patient II-3) and also her father (patient I-1) developed psoriasis, but no ichthyosis, based on clinical and histological evaluation of the lesions. This is the first observation of psoriasis in NLSD. In the compound heterozygous patient analogous, but less-pronounced lipid deposits, could be found in the basal epidermal layers as in patients with Chanarin–Dorfman disease.24 In the presence of obvious lipid storage in the different layers of skin in patient II-3, and increased lipid storage in patient I-1, we cannot rule out that psoriasis is related to defective lipolysis in these patients.
Our index patient with compound heterozygosity for mutations in the PNPLA2 gene is one of the few individuals surviving to adulthood with cardiac intervention with this novel type of NLSD showing symptomatic multisystem involvement and insulin resistance. Besides the classic presentation of progressive myopathy, cardiac involvement and insulin resistance, she demonstrates hearing loss and recurrent infections. There seems to be no liver involvement including fat storage, however, the significance of the liver adenoma is not yet clear. An interesting observation is the high frequency of ear infections (in the age range of 2 to 7 years/ 3–5 monthly) in the index patient with compound heterozygous mutation. This might be due to lipid storage in granulocytes and lymphocytes, however, no cellular or humoral immune deficiency could be demonstrated in our patients. Even more intriguing is the decrease in frequency of infections as she got older, which needs further explanation.
In our carrier family members, we could detect a significant TG storage not only in white blood cells but also in fibroblasts of the p.P195L and the p.Frameshift319* mutation carriers compared with healthy controls. These mutations have been previously shown to be inactive (p.P195L) or to exhibit strongly reduced LD association (p.Frameshift319*).13 Mutant variants lacking the C-terminal domain retain in vitro lipase activity, which is located in the intact N-terminal region containing a patatin domain and the catalytic site. The C-terminal domain contains a hydrophobic region, which is essential for LD binding.13, 25
PNPLA2 catalyzes the initial step in the breakdown of intracellular TGs24 and, as expected; PNPLA2-defective patients showed impaired degradation of cytoplasmic TGs. Experimental knockout of PNPLA2 in mice leads to cytoplasmic accumulation of TGs in multiple tissues.26 Interestingly, ATGL-deficient mice exhibit a strong decrease in PPARα target gene expression tissues with high-energy consumption, such as cardiac muscle and liver. As PPARs have a prominent role in the transcriptional regulation of genes involved in FA uptake and oxidation, ATGL deficiency is associated with impaired mitochondrial respiration. Reconstituting normal PPAR target gene expression by treatment with PPARα agonist reverses the mitochondrial defect, reduced fat accumulation in cardiac muscle and liver, and prologs life span of mice.27 It remains to be investigated whether PPAR agonists, such as fibrates, can improve myopathy and counteract TG accumulation in NLSD patients.
As the initial reports on PNPLA2-related NLSD, novel patients have been reported, with milder forms of the disease.5, 11, 12, 14, 15 One might question whether the disorder is not more common than previously thought, and the diagnosis is missed in some of these milder patients owing to missing the diagnostic sign of Jordans’ bodies (due to variable percentage of droplets in blood smear or the use of automated blood cell analysis) without specifically looking for the vacuoles by electron microscopy.
In summary, our data on increased TG content in muscle and in cultured fibroblasts of carriers implicate that the wild-type allele cannot fully compensate the mutated dysfunctional allele of PNPLA2 leading to defective TG catabolism and mild myopathy. Based on clinical and metabolic features of comparable severity expressed in both heterozygous p.P195L and p.Frameshift319* mutation carriers, we suggest screening patients with a mild form of lipid myopathy and also patients with unexplained hearing loss for the presence of Jordan’s bodies and PNPLA2-related NLSD.
Change history
18 July 2013
This article has been corrected since online publication and a corrigendum also appears in this issue.
References
Chanarin I, Patel A, Wills EJ, Andrews TM, Stewart G : Neutral lipid storage disease: a new disorder of lipid metabolism. Br Med J 1975; 1: 553–555.
Radom J, Salvayre R, Negre A, Maret A, Douste-Blazy L : Metabolism of neutral lipids in cultured fibroblasts from multisystemic (or type 3) lipid storage myopathy. Eur J Biochem 1987; 164: 703–708.
Jordans GH : The familial occurrence of fat containing vacuoles in the leukocytes diagnosed in two brothers suffering from dystrophia musculorum progressiva (ERB). Acta Med Scand 1953; 145: 419–423.
Fischer J, Lefevre C, Morava E et al: The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet 2007; 39: 28–30.
Chen J, Hong D, Wang Z, Yuan Y : A novel PNPLA2 mutation causes neutral lipid storage disease with myopathy (NLSDM) presenting muscular dystrophic features with lipid storage and rimmed vacuoles. Clin Neuropathol 2010; 29: 351–356.
Lefevre C, Jobard F, Caux F et al: Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am J Hum Genet 2001; 69: 1002–1012.
Hirano K, Ikeda Y, Zaima N, Sakata Y, Matsumiya G : Triglyceride deposit cardiomyovasculopathy. N Engl J Med 2008; 359: 2396–2398.
Akiyama M, Sawamura D, Nomura Y, Sugawara M, Shimizu H : Truncation of CGI-58 protein causes malformation of lamellar granules resulting in ichthyosis in Dorfman-Chanarin syndrome. J Invest Dermatol 2003; 121: 1029–1034.
Campagna F, Nanni L, Quagliarini F et al: Novel mutations in the adipose triglyceride lipase gene causing neutral lipid storage disease with myopathy. Biochem Biophys Res Commun 2008; 377: 843–846.
Kobayashi K, Inoguchi T, Maeda Y et al: The lack of the C-terminal domain of adipose triglyceride lipase causes neutral lipid storage disease through impaired interactions with lipid droplets. J Clin Endocrinol Metab 2008; 93: 2877–2884.
Akman HO, Davidzon G, Tanji K et al: Neutral lipid storage disease with subclinical myopathy due to a retrotransposal insertion in the PNPLA2 gene. Neuromuscul Disord 2010; 20: 397–402.
Reilich P, Horvath R, Krause S et al: The phenotypic spectrum of neutral lipid storage myopathy due to mutations in the PNPLA2 gene. J Neurol 2011; 258: 1987–1997.
Schweiger M, Schoiswohl G, Lass A et al: The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J Biol Chem 2008; 283: 17211–17220.
Akiyama M, Sakai K, Ogawa M, McMillan JR, Sawamura D, Shimizu H : Novel duplication mutation in the patatin domain of adipose triglyceride lipase (PNPLA2) in neutral lipid storage disease with severe myopathy. Muscle Nerve 2007; 36: 856–859.
Ohkuma A, Nonaka I, Malicdan MC et al: Distal lipid storage myopathy due to PNPLA2 mutation. Neuromuscul Disord 2008; 18: 671–674.
Lass A, Zimmermann R, Haemmerle G et al: Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab 2006; 3: 309–319.
Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL : ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep 2006; 7: 106–113.
Coassin S, Schweiger M, Kloss-Brandstätter A et al: Investigation and functional characterization of rare genetic variants in the adipose triglyceride lipase in a large healthy working population. PLoS Genet 2009; 6: e1001239.
Grönke S, Mildner A, Fellert S et al: Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab 2005; 1: 323–330.
Kurat CF, Natter K, Petschnigg J et al: Obese yeast: triglyceride lipolysis is functionally conserved from mammals to yeast. J Biol Chem 2006; 281: 491–500.
Villena JA, Roy S, Sarkady-Nagy E, Kim KH, Sul HS : Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem 2004; 279: 47066–47075.
Zechner R, Strauss JG, Haemmerle G, Lass A, Zimmermann R : Lipolysis: pathway under construction. Curr Opin Lipidol 2005; 16: 333–340.
Tack CJ, Ong MK, Lutterman JA, Smits P : Insulin-induced vasodilatation and endothelial function in obesity/insulin resistance. Effects of troglitazone. Diabetologia 1998; 41: 569–576.
Demerjian M, Crumrine DA, Milstone LM, Williams ML, Elias PM : Barrier dysfunction and pathogenesis of neutral lipid storage disease with ichthyosis (Chanarin–Dorfman Syndrome). J Invest Dermatol 2006; 126: 2032–2038.
Cornaciu I, Boeszoermenyi A, Lindermuth H et al: The minimal domain of adipose triglyceride lipase (ATGL) ranges until leucine 254 and can be activated and inhibited by CGI-58 and G0S2, respectively. PLoS One 2011; 6: e26349.
Haemmerle G, Lass A, Zimmermann R et al: Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006; 312: 734–737.
Haemmerle G, Moustafa T, Woelkart G et al: ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat Med 2011; 17: 1076–1085.
Acknowledgements
We thank the family. We acknowledge Professor Dr C Tack for performing the insulin clamp test and Mrs L Eshuis for preparing the electron microscopy figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest
Rights and permissions
About this article
Cite this article
Janssen, M., van Engelen, B., Kapusta, L. et al. Symptomatic lipid storage in carriers for the PNPLA2 gene. Eur J Hum Genet 21, 807–815 (2013). https://doi.org/10.1038/ejhg.2012.256
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2012.256
Keywords
This article is cited by
-
Genome-wide association meta-analysis identifies 17 loci associated with nonalcoholic fatty liver disease
Nature Genetics (2023)
-
Genetics of Familial Combined Hyperlipidemia (FCHL) Disorder: An Update
Biochemical Genetics (2022)
-
Neutral lipid storage disease with myopathy in China: a large multicentric cohort study
Orphanet Journal of Rare Diseases (2019)
-
Inborn errors of cytoplasmic triglyceride metabolism
Journal of Inherited Metabolic Disease (2015)