Abstract
One of the key signals regulating peripheral myelin formation by Schwann cell is the activation of the transcription factor NF-κB. Yet, whether NF-κB exerts similar functions in central myelin formation by oligodendrocytes remains largely unknown. We previously reported white matter abnormalities with unusual discordance between T2 and FLAIR sequences in a patient with intellectual disability and defective NF-κB signalling. These observations prompted us to hypothesise that NF-κB signalling may have a role in the axon myelination process of central neurons. We report here on five male patients with Xq28 duplications encompassing MECP2, three of which presented white matter anomalies on brain MRI. Array-CGH and FISH analyses demonstrated that brain abnormalities correlate with additional copies of the IKBKG, a gene encoding a key regulator of NF-κB activation. Quantitative RT-PCR experiments and κB-responsive reporter gene assays provide evidence that IKBKG overexpression causes impaired NF-κB signalling in skin fibroblasts derived from patients with white matter anomalies. These data further support the role of NF-κB signalling in astroglial cells for normal myelin formation of the central nervous system.
Similar content being viewed by others
Introduction
NF-κB is a ubiquitously expressed dimeric molecule that regulates the expression of a variety of genes and has a key role in a number of cellular processes such as innate and adaptive immunity, cellular proliferation, apoptosis and development.1 Inducible NF-κB activation depends on phosphorylation-induced proteosomal degradation of the inhibitor of NF-κB proteins, which retain inactive NF-κB dimers in the cytosol in unstimulated cells. The majority of the diverse signalling pathways that leads to NF-κB activation converge on the IκB kinase (IKK) complex, which is responsible for IκB phosphorylation, leading to its degradation and thus allowing nuclear translocation of the NF-κB complexes.
Myelin is an electrically insulating material that forms a layer, the myelin sheath, around the axon of a neuron. It is essential for the proper functioning of the nerve fibres in both the central nervous system (CNS) and the peripheral nervous system (PNS).2 Peripheral myelin formation during development is initiated by specific signals from axons to Schwann cells, and compelling evidence demonstrated that NF-κB orchestrates this complex process. NF-κB activity in the sciatic nerve of the developing rodents reaches a peak during the period when Schwann cells were actively myelinating axons.3 Moreover, blocking NF-κB activity impairs Schwann cell differentiation and myelination in vitro. Finally, the production of myelin is drastically reduced in the neurons of the dorsal root ganglia cultured from mice lacking the p65 subunit of NF-κB.
In humans, myelination in the CNS is a long-lasting process that starts during the fourteenth week of foetal development with only little amounts of myelin at the time of birth.4, 5 The process of myelination accelerates during infancy and will be pursued until adolescence. Brain myelin formation during development is initiated by, as yet, poorly identified signals from axons to oligodendrocytes. Reports linking white matter anomalies, which consists mostly of axons, their myelin sheaths and supporting oligodendrocytes, to defective pathways may thus allow a deeper understanding of how CNS myelination is regulated.
We previously reported three brothers in which intellectual disability (ID) associated with mild microcephaly and truncular obesity resulted from the loss-of-function mutation (p.R570X) of the TRAPPC9 gene (MIM 611966).6 Interestingly, brain MRI in affected sibs showed white matter abnormalities with unusual discordance between T2 and FLAIR sequences (ie, normal myelin signal on T2 sequence while FLAIR sequence shows important white matter abnormalities at the sus-tentorial level). So far, a total of 24 patients from seven distinct families have been reported with AR-NSID linked to TRAPPC9.7, 8, 9, 10, 11 In addition to microcephaly, obesity, early onset hypotonia and moderate-to-severe ID, white matter abnormalities is a constant finding in these patients.
TRAPPC9 (also known as NIBP, NIK and IKKβ binding protein) encodes a protein involved in the NF-κB signalling pathway and in nerve growth factor-induced neuronal differentiation.12 Consistently, impaired NF-κB signalling was observed in cultured skin fibroblasts of the index patient carrying the p.R570X TRAPPC9 null mutation. These observations prompted us to hypothesise that NF-κB signalling may have a role in the axon myelination process of the central neurons. To further test this hypothesis, we investigated NF-κB signalling in patients carrying Xq28 duplications of different sizes and associated or not with white matter anomalies.
Patients and methods
Patients
Five patients carrying an Xq28 duplication are reported in this study. Institutional research ethic approval and written consent were obtained for all the participants in the study.
Patient 1 is the first child of healthy non-consanguineous parents, born after an uneventful pregnancy with birth weight (BW) 2710 g and Apgar score 10. Shortly after birth, the boy presented hypotonia with delayed psychomotor development; he sat at 16–18 months and did not walk at 27 months. At 17 months he developed tonic-clonic seizures. The clinical examination at 27 months showed: weight 12.4 kg (−0.5 DS), height 80.5 cm (−2.5 DS), occipitofrontal circumference 46.5 cm (−2.5 DS), mild dysmorphic features (open, carp-shaped mouth, epicanthal folds, anteverted nares, tapering fingers), cryptorchidism, bilateral pyramidal syndrome, speech delay, and drooling. At this age, coronal Flair images showed an important delay of myelination of the white matter compared with age-matched normal controls while the coronal T2 images of the same affected boy showed no evident abnormalities of the white matter compared with normal controls indicating discordance between the FLAIR and T2 sequences (Figures 1g–j).
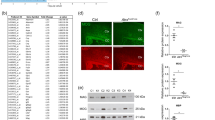
Brain MRI of patients 1–3 and of age-matched controls. (a) Coronal Flair weighted images of patient 2 at 6.5 years show an important and extensive hyperintensities of the periventricular and subcortical white matter compared with a 5.5 year-old normal control (e). By contrast, the axial T2-weighted FSE images of the same boy (b) show no evident abnormalities compared with normal control (f). Coronal Flair (c) and axial T2 (d) weighted images of patient 3 show normal matter compared with same age normal control (e, f). Coronal Flair weighted FSE images of the patient 1 at 2.9 year-old (g) show an important delayed of myelination of the white matter compared with a 2.5 year-old normal control (h). By contrast, the coronal T2-weighted FSE images of the same patient (i) show no evident abnormalities of the white matter compared with normal control (j).
Array-CGH identified a 8.6 Mb Xq27.3-q28 submicroscopic duplication caused by a de novo unbalanced translocation t(X;8)(q27.3-q28;qter) as further demonstrated by FISH analysis with the probe RP11-119A22. No loss of chromosome 8 material was detected (data not shown).
Patient 2 is the second child of healthy non-consanguineous parents. He was born at term with normal birth parameters after an uneventful pregnancy (BW: 3100g). He presented hypotonia, severe gastro-oesophageal reflux, bronchiolitis and pulmonary infections, which revealed an immunoglobulin deficit in IgM and IgG2, delayed motor and intellectual development (walked independently at 26 months of age) and poor social interactions. He was first seen at 6 years of age. Growth parameters and head circumference were within the normal range with weight on the 75th centile, height on the 50th centile and OFC on the 50th centile. He could speak some single words and remained hypotonic and ataxic with the absence of spasticity and presented stereotypic movements (hand flapping). Clinical examination showed exotropia, a small open mouth with drooling and slender hands and feet. He had a mild kyphosis. Genitalia were normal. Coronal Flair images showed an important and extensive hyperintensities of the periventricular and subcortical white matter compared with age-matched normal controls. On the other hand, the axial T2 of the same patient showed no obvious abnormalities compared with normal controls revealing discordance between the Flair and the T2 sequences (Figures 1a and b). Array-CGH identified an Xq28 microduplication subsequently confirmed by FISH analysis with the probe RP11-119M22 and inherited from the mother. His older brother had a similar clinical presentation with even more delayed motor skills due to spasticity and poorer social interactions. His brain MRI revealed similar Flair and T2 sequences discordance. He also carried the duplication as demonstrated by FISH analysis.
Patient 3 is the second child of healthy non-consanguineous parents. An undiagnosed X-linked ID syndrome segregated in the maternal family with three affected boys in two generations. He was born at term with normal birth parameters after an uneventful pregnancy (BW: 3125 g; BL: 48 cm; OFC: 35 cm). He presented hypotonia, severe chronic constipation, delayed motor and intellectual development (walked independently at 26 months of age) with limited social interactions and anxiety, bronchiolitis and pulmonary infections from 1 year of age, and epilepsy from 5 years of age. He was first seen at 5.5 years. Head circumference was on the 97th centile while weight and height were on the 75th centile. He was hypotonic, ataxic and spastic. Language was limited to about 10 words. Clinical examination showed exotropia, a small mouth, slender hands and feet, brisk tendon reflexes and drooling. Genitalia and spine were normal. No abnormalities were noted on brain MRI in both FLAIR and T2 sequences (Figures 1c and d).
A MECP2 duplication was clinically suspected and confirmed by FISH analysis with the probe RP11-119A22. The duplication was inherited from the mother.
Patient 4 was born after an uneventful pregnancy as the first child to healthy non-consanguineous parents. Family history was negative regarding congenital malformations and ID. At birth he was very hypotonic with feeding problems. His development was severely delayed, with sitting at the age of 2 years and walking with support at the age of 3 years and half. He did not develop any active speech but understands simple tasks. Repetitive behaviour and hand flapping when he is excited can be seen. At the age of 6 months he developed absence epilepsy responding well to Depakine. His major problems were the recurrent infections occurring since the first months of life, necessitating almost continuous antibiotic therapy, frequent hospitalisations and ventilation for 1 week at the age of 6 years because of a serious pneumonia. At the age of 7 years he walked again with support, his gait was broad based with eversion of the feet. He was still very hypotonic in the trunk and did not show any spasticity in the limbs. He made good eye contact with his care takers and had a happy character. Growth was normal. His face was hypotonic with tented upper lip and drooling. He could not feed himself. No abnormalities were noted on brain MRI in both FLAIR and T2 sequences.
X-chromosome array-CGH identified a small Xq28 microduplication in patient HT previously reported in the paper by Bauters et al.13
Patient 5 was reported previously as patient IV:2 from family 1 by Vandewalle et al.14 Briefly, this patient presented with delayed psychomotor development, tiptoeing and mild atactic gait. Cognitive evaluation showed a moderate ID, with a total IQ of 50. He had normal social interaction, and no behavioural problems have been reported. Array-CGH revealed a 317-kb-long microduplication at Xq28, between 153.218 and 153.535 Mb.
Neuroimaging methods
Anatomical MRI was performed with a 1.5-T (Signa General Electric Company, Fairfield, CT, USA) scanner using the following sequences: 3D T1-weighted FSPGR sequence, axial FSE T2-weighted imaging and coronal FLAIR sequences. Additional imaging sequences were occasionally obtained, including coronal T2 FSE or axial FLAIR sequences. The same paediatric neuroradiologist reviewed all brain images.
Quantitative RT-PCR (qRT-PCR) and stimulation assays
Reverse transcription was performed with the qSCRIPT cDNA SuperMix (QUANTA Biosciences, Gaithersburg, MD, USA) and the quantitative PCR with the KIT SYBR GREEN FASTMIX ROX (QUANTA Biosciences) on a 7300 Real-Time PCR System (Life Technologies Corporation, Carlsbad, CA, USA). The following primers were used: 5′-GAGCTCCGAGATGCCATC-3′ and 5′-CTCAGCCATCTGCTGCTG-3′ for IKBKG; 5′-AGCCCGTGCAGCCATCAGCC-3′ and 5′-CTTCCCAGGACTTTTCTCCA-3′ for MECP2; 5′-GAGTCAACGGATTTGGTCGT-3′ and 5′-TTGATTTTGGAGGGATCTCG-3′ for GAPDH. IKBKG and MECP2 transcripts levels were normalised to GAPDH mRNA.
Stimulation with TNF-α (10 ng/ml) was done on skin fibroblast cells from patient 2 and two independent controls who were sex and age-matched. TNF-α stimulation induces transcription of the c-IAP2 target gene. The amount of c-IAP2 transcript was evaluated by qRT-PCR using the following primers: 5′-GGTTCTTCTTCATGAAAGAAATG-3′ and 5′-CAGAAGATGTTTCAGATCTACCAG-3′.
Luciferase assays
Forty thousand SH-SY5H cells were co-transfected with 200 ng of a plasmid containing a κB-luciferase firefly reporter gene under the control of six NF-κB promoter sites, 100 ng of a plasmid containing luciferase Renilla construct under the control of a SV40 promoter and an increase amount of human IKBKG expression vector. The empty pcDNA3.1 vector was used to balance the transfected DNA concentration. The transfection was performed with Lipofectamine LTX and Plus Reagent (Life Technologies Corporation). Twenty-four hours after transfection, cells were stimulated for 5 h with 10 ng/ml of TNF-α and luciferase activity was measured in a luminometer using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
Results
IKBKG duplication in patients with white matter anomalies
Retrospective search for patients with cerebral MRI showing similar discordance between T2 and FLAIR sequences identified two patients originally diagnosed with a MECP2 duplication syndrome (patients 1 and 2). About 120 affected males with MECP2 gene duplication ranging from 0.3 to 8.0 Mb have been reported to date. Many children exhibit delayed myelination of the cerebral white matter at the posterior part of the cerebral hemispheres, yet, these abnormalities are not constant features and data are not available regarding T2/FLAIR discordance.15, 16, 17, 18, 19, 20 We hypothesised that such neuroradiological variability might correlate with the variable extent of Xq28 duplication and abnormal expression of other(s) gene(s) located within the duplicated region.
Based on our previous results on TRAPPC9 mutations, the gene encoding the inhibitor of kappa light polypeptide (IKBKG, MIM 300248, also known as NF-κB essential modulator) emerged as a strong candidate. Indeed, this gene, located 400 kb distal to MECP2, encodes the regulatory subunit of the IKK kinase complex.
To investigate whether IKBKG was duplicated we performed metaphase and interphase FISH analyses on leucocytes using the bacterial artificial chromosome clone RP11-103M23, which encompasses the IKBKG gene. Results demonstrated duplicated IKBKG in patients 1 and 2 but not in patients 3 and 4, suggesting a link between IKBKG duplication and white matter anomalies.
To further support our observations, we looked at the brain MRI of patient 5 who carries a recurrent 0.3 Mb-long Xq28 duplication identified in three X-Linked ID families and one sporadic ID patient.14 The aneusomic chromosomic fragment includes 18 annotated genes including IKBKG. MRI showed white matter abnormal hyperintense signals on both FLAIR and T2-weighted sequences and thin corpus callosum.
Effects of IKBKG duplication on gene expression
To evaluate the consequences of IKBKG duplication on the corresponding gene expression, we extracted and reverse transcribed RNA from cultured skin fibroblasts of patient 2. As expected, qRT-PCR demonstrated a two-fold overexpression of the MECP2 transcript. In addition, these cells also displayed an increased level of the IKBKG transcript (Figure 2a). Similarly, an increased level of IKBKG transcript was also observed in lymphoblastoid cell lines derived from patient 5 compared with controls (Figure 2b).
(a) qRT-PCR analysis of IKBKG and MECP2 transcripts in fibroblast cells from two controls (grey bars) and patient 2 (black bars). Data are normalised to GAPDH mRNA. Means±SD are given (n=5 independent experiments). ***Significance of difference with control values (Student’s test), P<0.01. (b) RT-qPCR of IKBKG and GDI1 transcripts in EBV-PBL cell lines from patient 5 (black bars) and three controls (grey bars). Normalised to GUSB. (c) Effect of IKBKG duplication on c-IAP2 expression. Analysis of TNF-α-induced c-IAP2 expression as detected by RT-PCR in skin fibroblast from patient 2. Data are normalised to GAPDH. Means±SD are given (n=3 independent experiments). ***Significance of difference with control values P<0.01 (Student’s test) and *significance of difference with control values P≤0.5 (Student’s test). (d) Overexpression of IKBKG inhibits NF-κB activation. SH-SY5H cells were co-transfected with a κB-luciferase reporter construct under the control of IgK-κB sites and increasing amount of IKBKG expression vector. The empty pcDNA3.1 vector was used to balance the transfected DNA concentration. Twenty-four hours after transfection, the cells were stimulated for 5 h with 10 ng/ml of TNF-α and luciferase activity was measured. Overexpression of IKBKG causes significantly reduced luciferase activity.
To confirm the pathogenic effect of IKBKG overexpression, we tested its impact on the induction of c-IAP2 (also known as BIRC3) mRNA expression, an endogenous κB-responsive gene, in response to TNF-α, a powerful activator of NF-κB signalling. Cultured fibroblasts from controls and patient 2 were TNF-α stimulated and c-IAP2 mRNA expression was measured by qRT-PCR. TNF-α treatment of control cells resulted in a 40-fold increased in c-IAP2 mRNA level as measured by qRT-PCR. By contrast, a 40% reduction of c-IAP2 mRNA induction was observed in cells carrying the IKBKG duplication when compared with two gender-matched controls (Figure 2c). These results support the hypothesis of an impaired NF-κB signalling in cells carrying the IKBKG duplication.
Finally, SH-SY5H human neuroblastoma cells were co-transfected with a luciferase reporter gene under the control of NF-κB binding sites and increasing amounts of a IKBKG expression vector. We observed that increased amount of IKBKG expression vector resulted in dose-dependent inhibition of a TNF-α induced NF-κB activation (Figure 2d).
Taken together, these data demonstrate that IKBKG duplication results in a NF-κB signalling defect and is correlated with white matter anomalies.
Discussion
Axon myelination is essential for axonal insulation and saltatory conduction of action potentials in the vertebrate nervous system. The remarkable multi-layered myelin sheath structure is achieved by wrapping of the plasma membrane of specialized glial cells, oligodendrocytes in the CNS and Schwann Cells in the PNS, around large-calibre axons. Among the signalling pathways that orchestrate axonal ensheathment in the PNS via Schwann cell differentiation to a myelinating phenotype, NF-κB was shown to be crucial for insulating axons. By contrast, the role of NF-κB in mediating oligodendrocyte maturation, myelination, de- and remyelination in the CNS has been less investigated. A recent study highlighted IKK2-dependent NF-κB signalling as a key pathogenic pathway during toxic CNS demyelination in mice.21 Yet, the exact contribution of astroglial NF-κB signalling to myelin formation during normal human CNS development remains largely unknown.
Our study supports the hypothesis of a correlation between cerebral white matter defects and impaired NF-κB signalling in humans. Taking into account our previous results on an autosomal recessive ID condition linked to TRAPPC9 mutation,6 these results strengthen the crucial role of NF-κB signalling for normal myelination process in the human CNS. Whether the MRI findings correspond to hypomyelination or dysmyelination remains unanswered. Reviewing a larger number of patients and regular MRI follow-up will be necessary to address this question.
Hypomyelination or myelination’s arrest is observed in other Mendelian and chromosomal disorders such as Pelizaeus–Merzbacher disease (PMD, MIM 312080), Allan-Herndon-Dudley syndrome (AHDS, MIM 300523) and 18qter deletion syndrome. PMD is an X-linked disorder caused by mutation of the gene encoding the proteolipid protein (PLP), one of the major myelin proteins.22 In this disorder, no or only a very little myelin is produced and high signal intensity is not restricted to FLAIR sequences alone, but also observed on T2-weighted images. AHDS is caused by mutations in a specific thyroid hormone transporter, the monocarboxylate transporter 8 (MCT8, SLC16A2). MRI imaging in these patients shows marked myelination delay that ressembles the one observed in patient 1 and 2. Finally, reduction of cerebral white matter and delayed myelination are the main histopathological findings in 18q deletion syndrome and discordance between T2 and FLAIR sequences are often observed. White matter abnormalities observed in this contiguous gene syndrome likely result from the loss of the gene encoding the myelin basic protein (MBP), the second major myelin protein.23
Myelinogenesis requires dramatic changes in the pattern of gene expression in a highly coordinated manner. Expression of myelin-specific genes is controlled by several families of transcription factors. Interestingly, functional NF-κB cis-elements have been identified on MBP and PLP gene promoters and TNF-α was found to modulate MBP transcription through NF-κB in a human oligodendroglioma cell line.24 Thus, impaired NF-κB signalling in the patients with IKBKG duplication could lead to misregulated MBP and/or PLP expression and myelination defect.
Beyond the demonstration that activation of NF-κB is an essential signal for the completion of myelination in CNS, these findings illustrate how brain MRI analysis can contribute to the understanding of pathophysiological mechanisms. Finally, they also suggest that it might be worth testing for NF-κB signalling integrity in patients with T2/Flair discordance.
References
Israel A : The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol 2010; 2: a000158.
Pereira JA, Lebrun-Julien F, Suter U : Molecular mechanisms regulating myelination in the peripheral nervous system. Trends in Neurosciences 2012; 35: 123–134.
Nickols JC, Valentine W, Kanwal S, Carter BD : Activation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formation. Nat Neurosci 2003; 6: 161–167.
Aggarwal S, Yurlova L, Simons M : Central nervous system myelin: structure, synthesis and assembly. Trends in Cell Biology 2011; 21: 585–593.
Kremer D, Aktas O, Hartung H-P, Küry P : The complex world of oligodendroglial differentiation inhibitors. Ann Neurol 2011; 69: 602–618.
Philippe O, Rio M, Carioux A et al. Combination of linkage mapping and microarray-expression analysis identifies NF-kappaB signaling defect as a cause of autosomal-recessive mental retardation. Am J Hum Genet 2009; 85: 903–908.
Abou Jamra R, Wohlfart S, Zweier M et al. Homozygosity mapping in 64 Syrian consanguineous families with non-specific intellectual disability reveals 11 novel loci and high heterogeneity. Eur J Hum Genet 2011; 19: 1161–1166.
Koifman A, Feigenbaum A, Bi W et al. A homozygous deletion of 8q24.3 including the NIBP gene associated with severe developmental delay, dysgenesis of the corpus callosum, and dysmorphic facial features. Am J Med Genet A 2010; 152A: 1268–1272.
Marangi G, Leuzzi V, Manti F et al. TRAPPC9-related autosomal recessive intellectual disability: report of a new mutation and clinical phenotype. Eur J Hum Genet 2012; e-pub ahead of print 2 May 2012 doi:10.1038/ejhg.2012.79.
Mir A, Kaufman L, Noor A et al. Identification of mutations in TRAPPC9, which encodes the NIK- and IKK-beta-binding protein, in nonsyndromic autosomal-recessive mental retardation. Am J Hum Genet 2009; 85: 909–915.
Mochida GH, Mahajnah M, Hill AD et al. A truncating mutation of TRAPPC9 is associated with autosomal-recessive intellectual disability and postnatal microcephaly. Am J Hum Genet 2009; 85: 897–902.
Hu WH, Pendergast JS, Mo XM et al. NIBP, a novel NIK and IKK(beta)-binding protein that enhances NF-(kappa)B activation. J Biol Chem 2005; 280: 29233–29241.
Bauters M, Van Esch H, Friez MJ et al. Nonrecurrent MECP2 duplications mediated by genomic architecture-driven DNA breaks and break-induced replication repair. Genome Res 2008; 18: 847–858.
Vandewalle J, Van Esch H, Govaerts K et al. Dosage-dependent severity of the phenotype in patients with mental retardation due to a recurrent copy-number gain at Xq28 mediated by an unusual recombination. Am J Hum Genet 2009; 85: 809–822.
Clayton-Smith J, Walters S, Hobson E et al. Xq28 duplication presenting with intestinal and bladder dysfunction and a distinctive facial appearance. Eur J Hum Genet 2009; 17: 434–443.
Kirk EP, Malaty-Brevaud V, Martini N et al. The clinical variability of the MECP2 duplication syndrome: description of two families with duplications excluding L1CAM and FLNA. Clin Genet 2009; 75: 301–303.
Lugtenberg D, Kleefstra T, Oudakker AR et al. Structural variation in Xq28: MECP2 duplications in 1% of patients with unexplained XLMR and in 2% of male patients with severe encephalopathy. Eur J Hum Genet 2009; 17: 444–453.
Meins M, Lehmann J, Gerresheim F et al. Submicroscopic duplication in Xq28 causes increased expression of the MECP2 gene in a boy with severe mental retardation and features of Rett syndrome. J Med Genet 2005; 42: e12.
Prescott TE, Rodningen OK, Bjornstad A, Stray-Pedersen A : Two brothers with a microduplication including the MECP2 gene: rapid head growth in infancy and resolution of susceptibility to infection. Clin Dysmorphol 2009; 18: 78–82.
Velinov M, Novelli A, Gu H et al. De-novo 2.15 Mb terminal Xq duplication involving MECP2 but not L1CAM gene in a male patient with mental retardation. Clin Dysmorphol 2009; 18: 9–12.
Raasch J, Zeller N, van Loo G et al. IkappaB kinase 2 determines oligodendrocyte loss by non-cell-autonomous activation of NF-kappaB in the central nervous system. Brain 2011; 134: 1184–1198.
Saugier-Veber P, Munnich A, Bonneau D et al. X-linked spastic paraplegia and Pelizaeus-Merzbacher disease are allelic disorders at the proteolipid protein locus. Nat Genet 1994; 6: 257–262.
Gay CT, Hardies LJ, Rauch RA et al. Magnetic resonance imaging demonstrates incomplete myelination in 18q- syndrome: evidence for myelin basic protein haploinsufficiency. Am J Med Genet 1997; 74: 422–431.
Huang CJ, Nazarian R, Lee J, Zhao PM, Espinosa-Jeffrey A, de Vellis J : Tumor necrosis factor modulates transcription of myelin basic protein gene through nuclear factor kappa B in a human oligodendroglioma cell line. Int J Dev Neurosci 2002; 20: 289–296.
Acknowledgements
We are grateful to the patients for their participation in the study. We sincerely acknowledge Elodie Bal for technical assistance and Sylvain Hanein for the gift of the SH-SY5 cell line. This study has been supported by the Centre National de la Recherche Scientifique (CNRS), the Agence Nationale de la Recherche (Grant NOANR-08-MNP-010) and the Ministère de la Recherche et de l’Enseignement Supérieur.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Philippe, O., Rio, M., Malan, V. et al. NF-κB signalling requirement for brain myelin formation is shown by genotype/MRI phenotype correlations in patients with Xq28 duplications. Eur J Hum Genet 21, 195–199 (2013). https://doi.org/10.1038/ejhg.2012.140
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2012.140
Keywords
This article is cited by
-
Duplication within two regions distal to MECP2: clinical similarity with MECP2 duplication syndrome
BMC Medical Genomics (2023)
-
Reversal of proliferation deficits caused by chromosome 16p13.11 microduplication through targeting NFκB signaling: an integrated study of patient-derived neuronal precursor cells, cerebral organoids and in vivo brain imaging
Molecular Psychiatry (2019)
-
Dysfunctional NF-κB and brain myelin formation
European Journal of Human Genetics (2014)