Abstract
The most common mutations found in FBN1 are missense mutations (56%), mainly substituting or creating a cysteine in a cbEGF domain. Other mutations are frameshift, splice and nonsense mutations. There are only a few reports of patients with marfanoid features and a molecularly proven complete deletion of a FBN1 allele. We describe the clinical features of 10 patients with a complete FBN1 gene deletion. Seven patients fulfilled the Ghent criteria for Marfan syndrome (MFS). The other three patients were examined at a young age and did not (yet) present the full clinical picture of MFS yet. Ectopia lentis was present in at least two patients. Aortic root dilatation was present in 6 of the 10 patients. In three patients, the aortic root diameter was on the 95th percentile and in one patient, the diameter of the aortic root was normal, the cross-section, however, had a cloverleaf appearance. Two patients underwent aortic root surgery at a relatively young age (27 and 34 years). Mitral valve prolapse was present in 4 of the 10 patients, and billowing of the mitral valve in 1. All patients had facial and skeletal features of MFS. Two patients with a large deletion extending beyond the FBN1 gene had an extended phenotype. We conclude that complete loss of one FBN1 allele does not predict a mild phenotype, and these findings support the hypothesis that true haploinsufficiency can lead to the classical phenotype of Marfan syndrome.
Similar content being viewed by others
Introduction
Marfan syndrome (MFS) is a dominant disorder mainly caused by mutations in the fibrillin-1 gene (FBN1) on chromosome 15. The estimated prevalence is about 1 in 10 000.1 The disorder has a very variable intra- and interfamilial expression. Different tissues and organs can be affected, with main features in the cardiovascular, skeletal, and ocular systems. Revised international criteria for the diagnosis were published in 1996 to facilitate the clinical diagnosis.2, 3
FBN1 mutations are detected in the majority of the patients fulfilling the clinical criteria, but also in incomplete phenotypes referred to as type 1 fibrillinopathies.4 Mutations in other genes have been reported to cause Marfan syndrome-related disorders, such as TGFBR1 and 2 in MFS type 2 (Mizuguchi et al5; Matyas et al6) and Loeys–Dietz syndrome7, 8 and MYH11 and ACTA2 in familial thoracic aortic aneurysms and dissections.9, 10
To date, over 600 mutations have been published in the Universal Marfan Database (UMD-FBN1; http://www.umd.be), but only a minority are recurring mutations. Missense mutations substituting or creating a cysteine in one of the calcium-binding EGF domains are most prevalent. Other mutations are frameshift, splice-site and nonsense mutations.11
Deletions of single and multiple exons can be detected using appropriate methods, such as multiplex ligation-dependent probe amplification (MLPA), cDNA or Southern blot analyses. Most of these deletions are associated with a severe or classical Marfan phenotype.12, 13, 14, 15, 16, 17 Only four reports are known of a molecularly proven whole-gene deletion of FBN1.18, 19, 20, 21 We describe 10 patients, including a family with five patients with whole-gene deletions, and show that complete loss of one FBN1 allele does not predict a mild phenotype. These findings support the hypothesis that true haploinsufficiency can lead to the classical phenotype of Marfan syndrome.
Patients and methods
Patients
We screened DNA samples of 300 patients with clinical features of MFS or a related phenotype by MLPA. All samples had been previously screened by DHPLC and no mutations in FBN1 were found. In one patient, chromosome analysis and array CGH performed for mental retardation screening revealed a deletion, including the FBN1 gene. In all patients, the size of the deletion was determined by SNP array analysis.
The clinical features of the patients are listed in Table 1. A more detailed description can be found in the Supplementary Material.
Multiplex ligation-dependent probe amplification
MLPA analysis was performed as described elsewhere22 with the SALSA MLPA kits P065 and P066 that contain probes for 53 of the 65 FBN1 exons (including exons 1 and 65) and all the 7 TGFBR2 exons, and reference probes widely spread over the genome (MRC-Holland, see http://www.mlpa.com).
PCR products were analyzed on a fluorescent capillary sequencer (ABI3130, Applied Biosystems, Torrence, CA, USA) using Genemarker software (Softgenetics Inc., State College, PA, USA).
Cytogenetic analysis (patients 8 and 9)
Conventional chromosome analysis was performed on phytohemagglutinin-stimulated lymphocytes from peripheral blood cultures using GTG banding according to standard protocols.
High-density microarray analyses, SNP arrays
The Affymetrix GeneChip Human Mapping 262K NspI array (Affymetrix, CA, USA) contains 262 000 25-mer oligonucleotides with an average spacing of ∼12 kb. An amount of 250 ng DNA was processed according to the manufacturer's instruction (www.Affymetrix.com). SNP copy number was assessed using the software program Copy Number Analyzer for Genechip (CNAG) Version 2.0 (see http://www.genome.umin.jp).23
Fluorescence in situ hybridization analysis
Fluorescence in situ hybridization (FISH) analysis was performed following the manufacturer's instructions using the BAC clone RP11-42K15 (Children Hospital Oakland Research Institute, Oakland, CA, USA).
Results
In nine patients, MLPA revealed reduced relative peak areas for all probes within the FBN1 gene, indicating a deletion of the entire FBN1 allele.
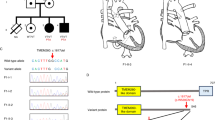
Five patients (patients 1–5) are part of one family (Figure 1). The parents of patients 1, 2 and 5 have no clinical features of MFS. However, in the mother, both MLPA and SNP array analysis showed lower intensity signals for the probes in the deleted area but higher signals than in the patients, suggesting a mosaic deletion.
Figure 2 shows the MLPA results in patient 2 and her mother. The other eight patients have MLPA results comparable with the results of patient 2. FISH analysis with a probe within the FBN1 gene confirmed the mosaic deletion in 21% of the totally 200 analyzed interphase nuclei. MLPA and FISH analysis in 200 interphase nuclei of the father showed a normal result (results not shown).
MLPA results of FBN1 in mother and daughter (patient 2) compared with healthy control (MLPA kit P065, MRC-Holland). The control probes are normalized to two copies. The probe signals for FBN1 relative to control probes and TGFBR2 probes show a single copy for FBN1 in patient 2. All other patients discussed in this paper show MLPA results comparable with patient 2. The healthy mother of patient 2 has reduced probe signals for all FBN1 probes, indicative of somatic mosaicism for the deletion. The mean (±SD) signals for all FBN1 probes were: 1.93±0.08 (control), 1.08±0.08 (case 2) and 1.71±0.05 (mosaic mother; P<10−15 compared with control, according to a two-tailed t-test). The color reproduction of this figure is available on the html full text version of the manuscript.
In three patients (patients 6, 8 and 9), the deletion occurred de novo. In one patient (patient 7), the parents were not tested for the deletion but appeared completely normal by clinical, ophthalmologic and cardiologic examination. The mother of patient 10 was not available for molecular testing.
In patient 8, the cytogenetic analysis revealed a de novo translocation between the long arms of chromosomes 12 and 15. Additional array CGH analysis, with a resolution of 1 Mb, detected a 4.9 Mb interstitial deletion at the translocation breakpoint of the long arm of chromosome 15 between the bands q21.1 and q21.2 (results not shown). The FBN1 gene is located in this region. At the translocation breakpoint of chromosome 12, no deletion was detected by array CGH or SNP array analysis. Conventional karyotyping of case 9 was performed as part of the mental retardation screening, showing a normal female karyotype. In the other patients, no standard cytogenetic analysis was performed.
For all probands, the size of the deletion was characterized by SNP array analysis. The results are depicted in Figure 3 and Table 2.
Position of the deletions on chromosome 15 (Ensemble release 53, March 2009) including the deletion described by Faivre et al19 The red bars underneath the chromosome depict the known protein-coding genes according to Ensemble release 53, March 2009. The names of the genes are written below. The horizontal colored lines show the size of the different deletions and their overlap. The color reproduction of this figure is available on the html full text version of the manuscript.
The clinical features of the patients are summarized in Table 1. Except for patients 8, 9 and 10, all patients fulfilled the Ghent criteria for Marfan syndrome. The young age of patients 8, 9 and 10 could explain why they do not yet present the full clinical picture of MFS. Ectopia lentis was present in patients 1 and 7. Patient 4 had questionable lens subluxation, and patient 6 had very mild lens subluxation of her right eye. Aortic root dilatation was present in 6 of the 10 patients. In patients 8, 9 and 10, the aortic root diameter was on the 95th percentile. In patient 6, the diameter of the aortic root was on the 50th percentile. The cross-section, however, had a cloverleaf appearance. Patients 2 and 7 underwent aortic root surgery at a relative young age (27 and 34 years, respectively). Mitral valve prolapse was present in 4 of the 10 patients, and billowing of the mitral valve in one. All patients had facial or skeletal features of MFS.
The two children with larger deletions (patients 8 and 9) had an extended phenotype with psychomotor retardation and additional features. Patient 8 was a 5-year old girl who presented with psychomotor retardation and hypotonia with severe motor delay at the age of 2.5 years. Patient 9 presented with psychomotor retardation with non-progressive ataxia. Apart from her marfanoid features, she had a very pale skin and hair without other ectodermal manifestations. She had facial dysmorphisms consisting of a brachycephalic skull, long philtrum, broad nose and prognathism.
Further details about the clinical manifestations of the 10 described patients are found in the Supplementary Information.
Discussion
There are several reports of deletions of the long arm of chromosome 15 involving chromosome band q21.1. However, in most of these reports, the deletion of FBN1 or the presence of marfanoid features are not discussed24, 25, 26, 27, 28, 29. In four reports, the deletion of FBN1 is confirmed by molecular techniques, with marfanoid features in three cases18, 19, 21 and absence of marfanoid features in one case, which could be due to the young age of this patient.20
In this study, we describe 10 patients with a deletion of an entire FBN1 allele. To our knowledge, this is the first series of complete FBN1 allele deletions published so far. These patients and three previously described sporadic patients18, 19, 21 have a Marfan phenotype due to pure haploinsufficiency. The phenotype of the patients in our series varies from mild features of MFS to the classical MFS phenotype. One family (patients 1–5) has a deletion encompassing only the FBN1 gene, whereas patients 6, 7, 8, 9 and 10 have much larger deletions spanning 1–9.4 Mb, with 9–46 genes, respectively (Figure 3 and Table 2). Patients 6, 7 and 10 have no other features than those that can be attributed to the deletion of FBN1. Patients 8 and 9 have psychomotor retardation and dysmorphic features. In addition, patient 9 has an extended phenotype with more severe neurological impairment, and lack of skin and hair pigmentation. The deleted genes Myosin 5A (MYO5A, MIM 160777) and RAS-associated protein (RAB27A, MIM 603868) could play a role in the phenotype of this girl. Mutations in MYO5A and RAB27A cause Griscelli syndrome types 1 and 2, respectively. These rare autosomal recessive disorders are characterized by partial albinism, immunological problems and/or neurological impairment. Further studies of these genes on the normal allele are pending. The three previously published case studies18, 19, 21 also have a deletion extending beyond the FBN1 gene. Faivre et al19 describes a teenage girl with a deletion of 2.97 Mb with some skeletal features of MFS and mitral valve prolapse, but absence of aortic root dilatation and ectopia lentis. Apart from language disabilities, she was not mentally retarded. The size of the deletion was characterized by array CGH, and 13 genes were found to be deleted including FBN1. In Figure 3, the size and position of this deletion is compared with the deletions described in this study. The patients described by Adès et al18 and Hutchinson et al21 have psychomotor retardation with additional features, probably due to haploinsufficiency of other genes. The size of the published deletions is unknown, but in the patient described by Hutchinson et al, the MFAP1 locus was deleted. That means that this deletion is extending more centromeric than our deletions. No further information is available about the breakpoints in these patients. Hutchinson et al21 found that in the deletion patient, the fibrillin-1 protein and mRNA levels were significantly higher than expected for a single FBN1 allele. They suggest that the clinical variability in MFS could be due to variable FBN1 expression from the normal allele. They compared their results with three members of one family with a premature termination codon (PTC) mutation, and showed that the variable expression in these individuals appeared to correlate with variability in FBN1 expression of the normal allele and not with variable rates of nonsense-mediated decay (NMD).
Apart from the PTC mutations where the phenotype will be due to partial haploinsufficiency caused by NMD and a dominant-negative effect of the fibrillin-1 molecules that escape NMD, few other mutations have been described, leading to a haploinsufficiency state. Milewicz et al30 described a patient with only skeletal features of MFS and a missense mutation in the FBN1 gene. This mutation cosegregated with tall stature in the family. The mutation disrupted the normal processing of one-half of the secreted profibrillin in fibrillin. Half the normal amount of fibrillin was shown to be deposited in structurally normal-appearing microfibrils. They hypothesized that this mutation mimics a null allele of FBN1 and leads to a milder phenotype, analogous to the null allele of COL1A1 which leads to the milder form of osteogenesis imperfecta.31, 32 Our results, however, show that haploinsufficiency of FBN1 is sufficient for the development of the full clinical expression of MFS with some carriers exhibiting severe features. For instance, two of our patients (patients 2 and 7) needed aortic surgery at a relatively young age (age 27 and 34, respectively). Additional evidence supporting the haploinsufficiency model are the two patients with classical MFS described by Mátyás et al16 with a deletion of the putative regulatory and promoter region of FBN1, resulting in complete loss of transcription of the corresponding allele. Both patients fulfilled the Ghent criteria with major manifestations in the skeletal and cardiovascular systems, but no ectopia lentis. The authors conclude that these two patients represent true haploinsufficiency.
Although no mouse model is known with a complete deletion of one FBN1 allele, the mouse model of Pereira suggests that there is a threshold of expression of the normal allele below which the abnormal phenotype will develop.33, 34 Mice with a heterozygous-targeted mutation leading to 15% expression of a normal product have no abnormal phenotype, whereas the mice with the same mutation on both alleles have severe abnormalities comparable with the neonatal MFS phenotype.
Judge et al35 used yeast artificial chromosome-based transgenesis to overexpress a disease-associated mutant form of human fibillin-1 (C1663R) on a normal mouse background. These mice showed no abnormalities, whereas a heterozygous comparable cysteine mutation in mice leads to the Marfan phenotype and histological changes as seen in heterozygous human. They showed that haploinsufficiency for the WT protein can be a significant factor in the pathogenesis of MFS when combined with an abnormal FBN1 allele. In keeping with the hypothesis of the critical contribution of haploinsufficiency, introduction of a wild-type transgene in the heterozygous mouse rescues the aortic phenotype.
How the lower production and deposition of fibrillin-1 will affect the TGFβ signaling pathway, and how it leads to the aortic and skeletal features is subject for debate. Recent evidence of the role of the TGFβ signaling pathway in the pathogenesis of MFS shows that fibrillin has a stabilizing effect on the latent TGFβ-binding protein-1 (LTBP-1) in the extracellular matrix (ECM)34, 35, 36, 37 LTBP1 plays a role in the release of TGFβ in the ECM. Mice with a Marfan phenotype and a centrally deleted FBN1 allele showed marked dysregulation of TGFβ activation and enhanced signaling.38 They hypothesize that deficiency of fibrillin-1 causes excessive amounts of active TGFβ to be liberated from the matrix. This might as well be the case in the patients with a deletion of the FBN1 gene. Increased TGFβ signaling is also shown in aortic tissue of patients with Loeys–Dietz syndrome.8 The exact mechanism, how changes in TGFβ signaling lead to such a specific phenotype, has still to be elucidated.
Ectopia lentis was present in at least two of our patients. The published patients with molecularly proven complete FBN1 allele deletions did not exhibit ectopia lentis.18, 19, 20, 21 None of the patients with a TGFBR2 or TGFBR1 mutation have ectopia lentis. We hypothesize that ectopia lentis in our patients is caused by the lower production of fibrillin-1 and not by perturbation of the TGFβ signaling. This is in keeping with the observation that mutations in LTBP2 (latent-transforming growth factor-β-binding protein 2) cause recessive eye abnormalities including ectopia lentis.39, 40 LTBP2 is the only member of the LTBP family not to bind to latent forms of TGFβ, and is thought to have an important structural role in the ciliary body together with fibrillin-1.41
In conclusion, our patients with a complete FBN1 allele deletion show that haploinsufficiency has a major contribution to the pathogenesis of MFS and can lead tot the whole spectrum of MFS. We hypothesize that the skeletal and aortic phenotype are caused by aberrant TGFβ signaling and the ocular phenotype by the lower production of the fibrillin-1 microfibrils.
References
Pyeritz RE : The Marfan syndrome. Annu Rev Med 2000; 51: 481–510.
Beighton P, De Paepe A, Danks D et al: International nosology of heritable disorders of connective tissue, Berlin, 1986. Am J Med Genet 1988; 29: 581–594.
De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE : Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet 1996; 62: 417–426.
Hayward C, Brock DJ : Fibrillin-1 mutations in Marfan syndrome and other type-1 fibrillinopathies. Hum Mutat 1997; 10: 415–423.
Mizuguchi T, Collod-Beroud G, Akiyama T et al: Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet 2004; 36: 855–860.
Matyas G, Arnold E, Carrel T et al: Identification and in silico analyses of novel TGFBR1 and TGFBR2 mutations in Marfan syndrome-related disorders. Hum Mutat 2006; 27: 760–769.
Loeys BL, Schwarze U, Holm T et al: Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006; 355: 788–798.
Loeys BL, Chen J, Neptune ER et al: A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005; 37: 275–281.
Zhu L, Vranckx R, Khau Van Kien P et al: Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet 2006; 38: 343–349.
Guo DC, Pannu H, Tran-Fadulu V et al: Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007; 39: 1488–1493.
Faivre L, Collod-Beroud G, Loeys BL et al: Effect of mutation type and location on clinical outcome in 1013 probands with Marfan syndrome or related phenotypes and FBN1 mutations: an international study. Am J Hum Genet 2007; 81: 454–466.
Blyth M, Foulds N, Turner C, Bunyan D : Severe Marfan syndrome due to FBN1 exon deletions. Am J Med Genet A 2008; 146A: 1320–1324.
Dietz HC, McIntosh I, Sakai LY et al: Four novel FBN1 mutations: significance for mutant transcript level and EGF-like domain calcium binding in the pathogenesis of Marfan syndrome. Genomics 1993; 17: 468–475.
Liu W, Schrijver I, Brenn T, Furthmayr H, Francke U : Multi-exon deletions of the FBN1 gene in Marfan syndrome. BMC Med Genet 2001; 2: 11.
Loeys B, Nuytinck L, Delvaux I, De Bie S, De Paepe A : Genotype and phenotype analysis of 171 patients referred for molecular study of the fibrillin-1 gene FBN1 because of suspected Marfan syndrome. Arch Intern Med 2001; 161: 2447–2454.
Matyas G, Alonso S, Patrignani A et al: Large genomic fibrillin-1 (FBN1) gene deletions provide evidence for true haploinsufficiency in Marfan syndrome. Hum Genet 2007; 122: 23–32.
Singh KK, Elligsen D, Liersch R et al: Multi-exon out of frame deletion of the FBN1 gene leading to a severe juvenile onset cardiovascular phenotype in Marfan syndrome. J Mol Cell Cardiol 2007; 42: 352–356.
Ades LC, Sullivan K, Biggin A et al: FBN1, TGFBR1, and the Marfan-craniosynostosis/mental retardation disorders revisited. Am J Med Genet A 2006; 140: 1047–1058.
Faivre L, Khau Van KP, Callier P et al: De novo 15q21.1q21.2 deletion identified through FBN1 MLPA and refined by 244K array-CGH in a female teenager with incomplete Marfan syndrome. Eur J Med Genet 2010; 53: 208–212.
Hiraki Y, Moriuchi M, Okamoto N et al: Craniosynostosis in a patient with a de novo 15q15-q22 deletion. Am J Med Genet A 2008; 146A: 1462–1465.
Hutchinson S, Furger A, Halliday D et al: Allelic variation in normal human FBN1 expression in a family with Marfan syndrome: a potential modifier of phenotype? Hum Mol Genet 2003; 12: 2269–2276.
Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G : Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res 2002; 30: e57.
Nannya Y, Sanada M, Nakazaki K et al: A robust algorithm for copy number detection using high-density oligonucleotide single nucleotide polymorphism genotyping arrays. Cancer Res 2005; 65: 6071–6079.
Fryns JP, de MA, van den Berghe H : Interstitial deletion of the long arm of chromosome 15. Ann Genet 1982; 25: 59–60.
Liehr T, Starke H, Heller A et al: Evidence for a new microdeletion syndrome in 15q21. Int J Mol Med 2003; 11: 575–577.
Mori MA, Rodriguez L, Pinel I, Casas JM, Diaz de BA, Martinez-Frias ML : Partial monosomy 15q due to de novo t(15;22)(q15;p11). Ann Genet 1987; 30: 246–248.
Pramparo T, Mattina T, Gimelli S, Liehr T, Zuffardi O : Narrowing the deleted region associated with the 15q21 syndrome. Eur J Med Genet 2005; 48: 346–352.
Shur N, Cowan J, Wheeler PG : Craniosynostosis and congenital heart anomalies associated with a maternal deletion of 15q15-22.1. Am J Med Genet A 2003; 120A: 542–546.
Yip MY, Selikowitz M, Don N, Kovacic A, Purvis-Smith S, Lam-Po-Tang PR : Deletion 15q21.1----q22.1 resulting from a paternal insertion into chromosome 5. J Med Genet 1987; 24: 709–712.
Milewicz DM, Grossfield J, Cao SN, Kielty C, Covitz W, Jewett T : A mutation in FBN1 disrupts profibrillin processing and results in isolated skeletal features of the Marfan syndrome. J Clin Invest 1995; 95: 2373–2378.
Herskowitz I : Functional inactivation of genes by dominant negative mutations. Nature 1987; 329: 219–222.
Byers PH : Inherited disorders of collagen gene structure and expression. Am J Med Genet 1989; 34: 72–80.
Dietz HC, Mecham RP : Mouse models of genetic diseases resulting from mutations in elastic fiber proteins. Matrix Biol 2000; 19: 481–488.
Pereira L, Lee SY, Gayraud B et al: Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci USA 1999; 96: 3819–3823.
Judge DP, Biery NJ, Keene DR et al: Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest 2004; 114: 172–181.
Isogai Z, Ono RN, Ushiro S et al: Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem 2003; 278: 2750–2757.
Pereira L, Andrikopoulos K, Tian J et al: Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nat Genet 1997; 17: 218–222.
Neptune ER, Frischmeyer PA, Arking DE et al: Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 2003; 33: 407–411.
Ali M, McKibbin M, Booth A et al: Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet 2009; 84: 664–671.
Desir J, Sznajer Y, Depasse F et al: LTBP2 null mutations in an autosomal recessive ocular syndrome with megalocornea, spherophakia, and secondary glaucoma. Eur J Hum Genet 2010; 18: 761–767.
Saharinen J, Keski-Oja J : Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta. Mol Biol Cell 2000; 11: 2691–2704.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Hilhorst-Hofstee, Y., Hamel, B., Verheij, J. et al. The clinical spectrum of complete FBN1 allele deletions. Eur J Hum Genet 19, 247–252 (2011). https://doi.org/10.1038/ejhg.2010.174
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2010.174
Keywords
This article is cited by
-
Identification of two novel large deletions in FBN1 gene by next-generation sequencing and multiplex ligation-dependent probe amplification
BMC Medical Genomics (2024)
-
An integration into the diagnostic workflow in a pediatric patient suspected of having Marfan syndrome
Egyptian Journal of Medical Human Genetics (2022)
-
An integrated clinical and molecular study of a cohort of Turkish patients with Marfan syndrome harboring known and novel FBN1 variants
Journal of Human Genetics (2021)
-
Parental mosaicism in Marfan and Ehlers–Danlos syndromes and related disorders
European Journal of Human Genetics (2021)
-
A novel splicing mutation in Marfan syndrome
International Journal of Legal Medicine (2020)