Abstract
A de novo 0.3 Mb deletion on 6p21.3 was detected by array-comparative genomic hybridization in a girl with mental retardation, drug-resistant seizures, facial dysmorphisms, gut malrotation and abnormal pancreas segmentation. Consistent with phenotypic manifestations is haploinsufficiency of SYNGAP1, which was recently demonstrated to cause non-syndromic mental retardation, and of the flanking genes CuTA, a likely modulator of the processing and trafficking of secretory proteins in the human brain, and hPHF1, involved in HOX gene silencing. Mutations of both CuTA and hPHF1 were never reported as causative of human diseases. Similarly, the present syndromic condition was not previously described and it can be regarded as a human model confirming the suggested biological properties of the genes included in the deletion interval. In addition, experimental evidence that SYNGAP1 and CuTA are involved in the secretory pathway in neurons, through glutamate and acetylcholinesterase signalling, prompted us to consider modulation of the glutamate pathway as target of a therapeutic strategy for seizure control.
Similar content being viewed by others
Main
Chromosome abnormalities, including submicroscopic rearrangements detected by array-comparative genomic hybridization (a-CGH), represent the most common cause of syndromic mental retardation. On the other hand, a consistent number of copy number variations (CNVs), either inherited or de novo, are identified in normal individuals, complicating the interpretation of quantitative changes of several genomic regions. Current criteria for characterizing them as polymorphisms or pathogenic imbalances include evaluation of the CNV size, 1 Mb being considered a threshold for benign variants, and gene content analysis.1, 2 In some cases, on the basis of the recurrence of chromosomal microdeletions in association with specific phenotypes, single genes have been identified as the underlying cause of syndromes with mental retardation and multiple congenital anomalies, such as the Mowat–Wilson3 and Pitt–Hopkins4 syndromes. In many instances, mental retardation is associated with morphological changes in dendritic spines, suggesting that altered synaptic plasticity functions as a common pathogenetic mechanism.5 This evidence prompted Hamdan et al6 to look for mutations in SYNGAP1, an autosomal gene encoding a component of the N-methyl-D-aspartate (NMDA)-receptor complex, which modulates synaptic plasticity, in non-syndromic mental retardation. Truncating mutations were identified in 3 of 94 mentally retarded subjects, all lacking physical dysmorphisms and malformations.
By a-CGH, we detected a de novo 0.3 Mb deletion in 6p21.3 in a 3-year-old girl with mental retardation and multiple congenital anomalies, notably pancreas segmentation and intestinal malrotation. Included in the deletion interval is SYNGAP1, as well as the flanking genes CuTA and hPHF1. Both SYNGAP1 and CuTA are likely involved in the secretory pathway in neurons.7, 8 HPHF1 belongs to the polycomb group of genes and it was recently reported as a key regulator of homeobox and developmental gene expression.9 Because of the potential role of these genes in brain plasticity and organ morphogenesis, we hypothesize that their haploinsufficiency explains the clinical findings in our patient, allowing us to define a new contiguous gene syndrome.
Case report
The proposita is a 5-year-old girl, the second child of healthy, non-consanguineous parents. Her older brother is healthy. She was born at 38 weeks by caesarean section after a pregnancy complicated by polyamnios. Birthweight was 3300 g (50–90th centile), length 47 cm (10–25th centile) and head circumference 35 cm (50th centile). The neonatal period was characterized by poor sucking, gastro-oesophageal reflux and generalized hypotonia. Psychomotor delay was observed during the first months of life. Feeding difficulties due to constipation and aerophagy caused postnatal growth delay since the age of 10 months. She experienced an episode of acute pancreatitis at 12 months and underwent surgery for a pancreatic cyst at 21 months. During the intervention, a malrotation of the upper gut was noted and, when examined with barium enema, a marked intestinal hypomobility was detected. At 18 months, a pancreas divisum was diagnosed by endoscopic retrograde cholangiopancreatography. Epilepsy started at the age of 3 months with myoclonic seizures, which were initially well controlled by clobazam. In the following few months, the patient experienced recurrent exacerbations of epilepsy, with polymorphic generalized seizures (myoclonic jerks, atonic attacks, atypical absences) that concurred with sleep abnormalities (derangement of sleep/wake rhythm, insomnia, sleep instability and fragmentation) and behavioural regression. EEG while awake and asleep showed multifocal abnormalities (slow spike-waves, spike- and polyspike-waves), which became subcontinuous during sleep and were reminiscent of the EEG features of the Lennox–Gastaut syndrome. Sodium valproate associated with carnitine was effective in controlling seizures and led to remarkable improvement in the developmental and behavioural disorders. However, after a few months, seizures relapsed, which prompted the use of phenobarbital and topiramate. The use of topiramate resulted in a prolonged seizure-free period. At the age of 4 years, the patient was able to utter a few words and walk unsupported. Her DQ (Griffiths Mental Developmental Scale) was 32 (mental age 17 months). EEG alterations were only moderately influenced by treatment. Although transient exacerbations of seizures still occurred, the general clinical conditions markedly improved after topiramate therapy.
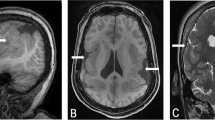
On physical examination at the age of 3 years, weight was 11.2 kg (third centile), length 86 cm (third centile) and OFC 48.4 cm (25th centile). A peculiar facial appearance was noted, consisting of frontal bossing, epicanthic folds, flat philtrum, tented upper lip and midface hypoplasia (Figure 1). Auricles were small and a lobular pit was noted, bilaterally. The midline furrow of the tongue was very deep and a supernumerary nipple was present on the right thorax. Feet were small with crowding of toes. The girl was severely mentally retarded: she could not walk unsupported, her social interaction was poor and inconsistent, and language was essentially absent; she tended to withdraw and activate Rett-like stereotypies. Brain MRI and an extensive neurometabolic workup were normal.
Molecular cytogenetics
By the R(RBG) banding method, an apparently normal 46,XX karyotype was detected. Array-CGH was performed using Agilent oligonucleotide-array kit 44B (Human Genome CGH Microarray Kit 44B; Agilent Technologies, Santa Clara, CA, USA), with an average resolution of about 75 kb, following the manufacturer's instructions. A de novo interstitial deletion spanning about 0.3 Mb of chromosome region 6p21.3 was detected, with distal breakpoint (last deleted probe A_14_P100360, first preserved probe A_14_P122500) at about 33.4 Mb from the 6p telomere and proximal breakpoint (last deleted probe A_14_P133907, first preserved probe A_14_P112116) at about 33.7 Mb from the 6p telomere. Included in the deletion interval are genes SYNGAP1, CuTA, and hPHF1.
Results were confirmed by FISH analysis on metaphase spreads with the BAC clone RP11.175A4 containing SYNGAP1 (Wellcome Trust Sanger Institute, Hinxton, Cambridge, UK). Results are summarized in Figure 2. FISH analysis with the same probe was carried out in both parents, with normal results. Patient's karyotype was 46,XX.arr cgh (A_14_P160360 → A_14_P133907) × 1 .ish del(6)(p21.3)(RP11.175A4 -)dn.
(a) Map of the deleted 300-kb -region within 6p21.3. Genes involved in the rearrangement are shown. Grey squares refer to genes not considered to be relevant for the phenotype. Black squares refer to pathogenic genes. (b) Graphical overview of the results obtained by array-CGH analysis. Included in the deletion interval (grey rectangle) are seven known genes. (c) FISH results on metaphase chromosomes with BAC probe RP11.175A4 containing SYNGAP1. Only one signal is present.
Discussion
We report on a previously undescribed association of severe mental retardation, hypotonia, seizures, facial dysmorphisms and abnormalities in gut rotation and pancreas segmentation. Causative of this complex disorder is a very small 6p21.3 deletion, spanning about 0.3 Mb, which was detected by a-CGH. Validation of the a-CGH results and genotype–phenotype correlations were possible on the basis of gene content analysis. Although a total of seven known genes are included in the deletion interval, we tentatively consider this condition as a contiguous gene syndrome caused by haploinsufficiency of three of these genes, namely, SYNGAP1, CuTA and hPHF1. Known pathogenic mutations in humans are limited to SYNGAP1. Truncating mutations of this gene were recently described by Hamdan et al6 in three patients with mental retardation and language delay, two of whom were also epileptic. SYNGAP1 is a component of the NMDA-receptor complex, which regulates the plasticity of excitatory synapses through glutamate signalling.10 Disruption of pathways modulating synaptic plasticity and spines morphogenesis appears to be a common mechanism in several forms of mental retardation.5, 11 Confirming the exclusive expression of SYNGAP1 in the brain, patients in the study by Hamdan et al lack physical anomalies. It is worth noting that the seizure disorder was reported to improve with valproate, which modulates the glutamate pathway.
In addition to mental retardation, language delay and epilepsy, our patient also presented with facial dysmorphisms and unusual abnormalities, including pancreas divisum and intestinal malrotation. We consider that hPHF1 hapoinsufficiency likely correlates with the altered morphogenesis. hPHF1 encodes a polycomb group protein involved in histone methylation and Hox genes silencing.9 It is the human homologue of Drosophila Pcl, which functions in silencing Hox genes in a tissue-specific manner. Interestingly, Pcl mutants show a stronger misexpression of Hox genes in the viscera mesoderm, as can be hypothesized in our patient.12
With respect to CuTA, haploisufficiency of this gene likely correlates with the seizure disorder, in addition to SYNGAP1. In fact, the mammalian protein CuTA seems to modulate the processing and trafficking of secretory proteins in the human brain. It was recently demonstrated that the mouse variants of CuTA affect the folding, oligomerization and secretion of acetylcholinesterase (AChE) into a catalytically active conformation, and several studies point to a role of AChE in the development of epilepsy.8 The inferred disruption, in our patient, of the glutamate pathway prompted us to consider anticonvulsant drugs modulating this pathway as prime drugs for seizure control. Indeed, valproate (transiently) and topiramate (more persistently) had a remarkable effect on seizure control and led to a general improvement in the developmental and behavioural disorders.
The deletion interval includes other genes, that is, KIFC1, ZBTB9, BAK1 and GGNBP1, which could also be expected to have a role in clinical manifestations. KIFC1 encodes a motor protein required for bipolar spindle formation. It may contribute to movement of early endocytic vesicles. The functional role of ZBTB9 is unknown. BAK1 (BCL-2 homologous antagonist/killer) induces programmed cell death by binding to, and antagonizing, the apoptosis repressor BCL2, and accelerates the opening of the mitochondrial voltage-dependent anion channel, which leads to a loss of membrane potential and release of cytochrome c. This protein also interacts with the tumour suppressor P53 after exposure to stress. GGNBP1 (gametogenin binding protein 1) is expressed specifically in germ cells from late primary spermatocytes to early round spermatids and it is likely involved in spermatogenesis. On the basis of their function and on clinical evidence, KIFC1, ZBTB9, BAK1 and GGNBP1 were considered to be irrelevant for the observed phenotype.
In spite of extensive experimental investigations, the function of CuTA and hPHF1 in humans is still unknown. Mutations or deletions of these genes have been decribed so far in association with human diseases. To the best of our knowledge, only one literature report deals with disruption of SYNGAP1, limited to three patients, as discussed above.6
Another patient with a 0.49 Mb deletion on 6p21.3, proximally contiguous to the deletion reported here and including SYNGAP1 only, is reported in the Decipher database (https://decipher.Sanger.ac.uk). This patient presented with mental retardation, hyperlaxity of joints and abnormal male genitalia. All together, these reports, including the present one, confirm that SYNGAP1 haploinsufficiency can cause mental retardation through loss-of-function gene mutations, which can be detected by gene sequencing, or by complete gene loss, which can only be detected by quantitative molecular cytogenetics techniques. We can speculate that the syndromic association we describe here represents a human model for downregulation of CuTA and hPHF1 as well.
This report supports the notion that correlating gene content analysis of CNVs detected by a-CGH with clinical signs can suggest the pathogenesis, and possibly a targeted treatment, of human diseases caused by chromosome imbalances.
References
Lee C, Iafrate AJ, Brothman AR : Copy number variations and clinical cytogenetic diagnosis of constitutional disorders. Nat Genet 2007; 39: S48–S54.
Koolen DA, Pfundt R, de Leeuw N et al: Genomic microarrays in mental retardation: a practical workflow for diagnostic applications. Hum Mutat 2009; 30: 283–292.
Mowat DR, Wilson MJ, Goossens M : Mowat-Wilson syndrome. J Med Genet 2003; 40: 305–310.
Amiel J, Rio M, de Pontual L et al: Mutations in TCF4, encoding a class I basic helix-loop-helix transcription factor, are responsible for Pitt-Hopkins syndrome, a severe epileptic encephalopathy associated with autonomic dysfunction. Am J Hum Genet 2007; 80: 988–993.
Purpura DP : Dendritic spine ‘dysgenesis’ and mental retardation. Science 1974; 186: 1126–1128.
Hamdan FF, Gauthier J, Spiegelman D et al: Mutations in SYNGAP1 in autosomal nonsyndromic mental retardation. N Engl J Med 2009; 360: 599–605.
Rumbaugh G, Adams JP, Kim JH et al: SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons. Proc Natl Acad Sci USA 2006; 103: 4344–4351.
Liang D, Nunes-Tavares N, Xie HQ et al: Protein CutA undergoes an unusual transfer into the secretory pathway and affects the folding, oligomerization, and secretion of acetylcholinesterase. J Biol Chem 2009; 284: 5195–5207.
Cao R, Wang H, He J et al: Role of hPHF1 in H3K27 methylation and Hox gene silencing. Mol Cell Biol 2008; 28: 1862–1872.
Kim MJ, Dunah AW, Wang YT et al: Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron 2005; 46: 745–760.
Laumonnier F, Cuthbert PC, Grant SG : The role of neuronal complexes in human X-linked brain diseases. Am J Hum Genet 2007; 80: 205–220.
Soto MC, Chou TB, Bender W : Comparison of germline mosaics of genes in the polycomb group of Drosophila melanogaster. Genetics 1995; 140: 231–243.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zollino, M., Gurrieri, F., Orteschi, D. et al. Integrated analysis of clinical signs and literature data for the diagnosis and therapy of a previously undescribed 6p21.3 deletion syndrome. Eur J Hum Genet 19, 239–242 (2011). https://doi.org/10.1038/ejhg.2010.172
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2010.172
Keywords
This article is cited by
-
Profiling Autism and Attention Deficit Hyperactivity Disorder Traits in Children with SYNGAP1-Related Intellectual Disability
Journal of Autism and Developmental Disorders (2023)
-
Clinical and behavioural features of SYNGAP1-related intellectual disability: a parent and caregiver description
Journal of Neurodevelopmental Disorders (2022)
-
Transcriptional signatures of participant-derived neural progenitor cells and neurons implicate altered Wnt signaling in Phelan-McDermid syndrome and autism
Molecular Autism (2020)
-
Identification of an individual with a SYNGAP1 pathogenic mutation in India
Molecular Biology Reports (2020)