Abstract
Axenfeld–Rieger syndrome (ARS) is a rare autosomal dominant disorder, which encompasses a range of congential malformations affecting the anterior segment of the eye. ARS shows genetic heterogeneity and mutations of the two genes, PITX2 and FOXC1, are known to be associated with the pathogenesis. There are several excellent reviews dealing with the complexity of the phenotype and genotype of ARS. In this study, we will attempt to give a brief review of the clinical features and the relevant diagnostic approaches, together with a detailed review of published PITX2 and FOXC1 mutations.
Similar content being viewed by others
In brief
-
Axenfeld–Rieger syndrome (ARS) is an umbrella term used to describe a variety of overlapping phenotypes, in which the major physical condition is the anterior segment dysgenesis of the eye.
-
Patients with ARS may also present systemic malformations with incomplete penetrance and variable expressivity. The major systemic features are mild tooth abnormalities (microdontia, hypodontia, oligodontia and adontia) and redundant periumbilical skin. Craniofacial dysmorphism such as maxillary hypoplasia, sensory hearing loss, hypertelorism and congenital heart defects may also be part of the clinical spectrum.
-
ARS is a dominantly inherited condition with genetic heterogeneity.
-
Mutations in the transcription factors, PITX2 and FOXC1, lead to ARS.
-
The mutations show great diversity from intragenic mutations (PITX2 and FOXC1) to submicroscopic deletions (PITX2 and FOXC1) or duplications (FOXC1), to chromosome rearrangements (PITX2).
-
There is no clear genotype–phenotype relationship but ARS patients with systemic changes usually have PITX2 mutations.
-
The underlying genetic defect is unknown in 60% of the cases and there are at least two more loci associated with ARS, but the genes involved are yet to be identified.
-
The major clinical concern is the risk of developing sight-threatening glaucoma, which is observed in 50% of the patients.
Introduction
Axenfeld–Rieger syndrome (ARS) is mainly characterised by anterior segment abnormalities (anterior segment dysgenesis, ASD) of the eye, and comprises a clinically and genetically heterogeneous group of conditions with a varying degree of developmental abnormalities involving both ocular and extraocular structures.1 Several classifications of anterior segment disorders have been suggested and the terminology used is quite complex. In this review, the general term ARS will be used for the conditions, Axenfeld anomaly, Axenfeld syndrome, Rieger anomaly and Rieger syndrome.2, 3 As our understanding of the embryology and genetics of the eye development increases, these terminologies and classifications may take new shapes. The other disorders of the anterior segment, such as Peters anomaly and iris hypoplasia/iridogoniodysgenesis anomaly/syndrome will be mentioned only briefly and the focus of the review will be mainly on the ARS.
Clinical features
The clinical features of ARS can be roughly divided into ocular and non-ocular (systemic) changes.
Ocular changes
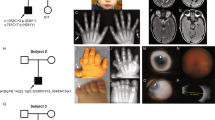
The ocular abnormalities observed in ARS affect mainly the iris, cornea and the chamber angle (Figure 1a–d).
Ocular changes observed in Axenfeld–Rieger syndrome. (a) Corectopia on the right eye (displaced pupil is shown by an arrow); (b) Polycoria (the extra hole on the iris is shown by an arrow); (c) Posterior embryotoxon, the prominent Schwalbe's line is indicated with arrows; (d) Iris strands bridging the chamber angle as seen on gonioscopy.
Iris Observation of iris changes is important in establishing the ARS diagnosis and these include thinning of the iris (hypoplasia), displacement of the pupil (corectopia) (Figure 1a) or hole formation in the iris mimicking multiple pupils (polycoria) (Figure 1b). The iris changes may be very subtle (only slight peaking of the pupil) and may seem to be normal without examination of the iridocorneal angle (gonioscopy). Dependent on the placement of the pupil corectopia and polycoria may cause photophobia and cosmetic problems.
Cornea The Schwalbe's line (the peripheral termination of Descemet's membrane and the anterior limit of the trabeculum) is prominent and displaced anteriorly (posterior embryotoxon) (Figure 1c). It appears as a white line on the posterior cornea, near limbus and can be observed in slit lamp examination and it is easily diagnosed on gonioscopy. Posterior embryotoxon is found in most ARS patients, but is not required for diagnosis.4 Approximately 15% of the general population has posterior embryotoxon, without increased risk of developing glaucoma.5 When posterior embryotoxon is identified in a patient with an anterior segment disorder, the first consideration should be ARS. Absence of other corneal abnormalities, such as megalocornea, sclerocornea and corneal opacity are the useful criteria in distinguishing ARS from other anterior segment disorders.
Chamber angle In ARS, a characteristic chamber angle appearance is observed and the iris strands bridge the iridocorneal angle to the trabecular meshwork (Figure 1d). The iris processes/strands may be attached to the Schwalbe's line and may have variable thickness. Presence of these changes should be examined with gonioscopy, whenever ARS is suspected.
Increased ocular pressure (IOP) leading to glaucoma is the major consequence of the eye dysgenesis observed in ARS, where approximately half of the patients develop secondary glaucoma.1 Glaucoma can develop in infancy, but usually occurs in adolescence or early adulthood. In some cases it can be observed after middle age. As ARS patients are at risk of glaucoma development throughout their lives, they should be examined annually for the changes in IOP and the optic nerve head.
Systemic findings
Axenfeld–Rieger syndrome patients may have accompanying systemic features. The most characteristic features are mild craniofacial dysmorphism (Figure 2), dental anomalies and redundant periumblical skin. The midface abnormalities include hypertelorism, telecanthus, maxillary hypoplasia with flattening of the mid-face, prominent forehead, and broad, flat nasal bridge. Dental abnormalities may be small teeth (microdontia) or fewer teeth than normal. In the abdominal region, a failure of involution of the skin resulting in redundant periumbilical skin can be seen and this may be mistaken for an umbilical hernia. Hypospadia in males, anal stenosis, pituitary abnormalities and growth retardation may also be found. Systemic changes other than these are usually not considered as the classical features of ARS.
(a) Clinical photographs of father (33 years) and son (3 years) affected with Axenfeld–Rieger syndrome. ( b) Female patient (two and half years). All the patients show similar morphological features, including telecanthus, broad nasal bridges and prominent lower lips. The father and the female patient have maxillary hypoplasia and additionally the female patient has dental anomalies with small crowns.
Other disorders with ASD
Idrees et al3 have suggested a very useful classification of anterior segment dysgeneses based on the underlying embryological abnormality. In this classification, diseases caused by abnormal neural crest migration and differentiation are termed anterior segment dysgeneses-neural crest and these include ARS, infantile/primary congenital glaucoma (PCG), iris hypoplasia/iridogoniodysgenesis anomaly/syndrome (IH, suggested as a single disorder2, 3), Peters anomaly, congenital hereditary endothelial dystrophy, sclerocornea and megalocornea. Anterior segment disorders of primarily non-neural crest origin include aniridia, which mainly affects the posterior iris.
Disorders of ASD, which should be considered in differential diagnosis of ARS, are IH, Peters anomaly and PCG. In IH iris hypoplasia and goniodysgenesis is present, but posterior embryotoxon or iris adhesions are not found.3 Peters anomaly is an ASD with central absence of the corneal endothelium, Descemet's membrane and posterior corneal stroma, leading to central corneal opacity, which is not present in ARS. Cataract may also be present in Peters anomaly.6 Patients with PCG have buphthalmos, goniodysgenesis and a high IOP causing corneal edema, photophobia and tearing, and embryotoxon or iris adhesions are normally not observed.
Embryology of the anterior segment
The anterior segment of the eye includes all the structures (cornea, iris and chamber angle) lying between the front surface of the cornea and the front surface of the vitreous. A developmental defect of these structures, which are derived from the neural crest cells, will lead to ASD. Neural crest cells are neuroectodermal cells, which migrate from the crest of the neural tube to several sites in the developing embryo. In the eye, neural crest cells migrate to the developing anterior chamber of the eye forming the keratocytes and corneal endothelium, iris stroma cells, melanocytes, trabecular meshwork and juxtacanalicular tissue.3, 7, 8 Developmental arrest that occurs late in gestation results in the neural crest cells being retained over parts of the iris and anterior chamber angle.3 This affects the anatomy of the anterior chamber angle, eventually effecting aqueous drainage and causing glaucoma as observed in ARS. In addition, contraction of the primordial layer causes iris stromal thinning, corectopia and hole formation, all of which are malformations observed in ARS. Retention of the neural crest cells may also result in iris hypoplasia, Peters anomaly, anirida, sclerocornea, megalocornea and primary congenital glaucoma.
Neural crest cells play an important role not only in the development of the anterior segment, but they also give rise to many structures, such as bone and cartilage of the skull, teeth and dermis, which may explain involvement of other organs in ARS.
Genetic basis of ARS
The conditions that comprise ARS are inherited in autosomal dominant manner with high penetrance. ARS is genetically heterogeneous and mutations in different genes lead to similar clinical conditions. ARS has been associated with mutations of two known genes: PITX2 (the pituitary homeobox 2 gene) at 4q259 and, FOXC1 (the forkhead box C1 gene, FKHL7) at 6p25.10, 11 A third locus was suggested by deletion of 13q14, supported by linkage analyses, but a disease-causing gene has not been identified yet.12, 13 In two isolated cases, deletion of the 16q23-q24 region14 and deletion of the PAX6 gene at 11p13,15 respectively, were related to ARS, but these findings were not supported by other studies. In ∼60% of patients the genetic defect in ARS is not known (not caused by mutation of an already identified gene).16 However, accurate estimation of the prevalence of mutations, is hampered by the fact that this is a rare condition and screening of large cohorts is not possible.
PITX2 and FOXC1
The two known ARS-associated genes, PITX2 and FOXC1, both encode developmental transcription factors (Table 1). Transcription factors are proteins, which regulate expression of downstream target genes through binding to specific DNA sequences and activating transcription. Transcription factors play an important role in orchestrating normal embryonic development and their expression is regulated precisely in temporal and spatial patterns.
The PITX2 gene encodes a homeodomain-containing transcription factor, which recognizes and binds to specific DNA sequences through the homeodomain, and functions as a transcription regulator during embryogenesis and development of different tissues of the anterior segment. The gene produces four mRNA transcripts (PITX2A-D), but a translation product of PITX2D has never been detected. The three other isoforms (A–C) differ at the N terminus, but they all include the 60-amino-acid homeodomain. They all have identical C termini with a conserved 14-amino-acid OAR domain (otp, aristaless and rax), which is predicted to mediate protein–protein interactions and self-inhibitory interactions with the N terminus.17 The smallest isoform, PITX2A (32 kDa), is the best studied with regards to ARS malformations and the PITX2 mutations reviewed in this study are described with regards to this isoform (Supplementary Figure 1).18
The FOXC1 gene is a member of the forkhead family of transcription factors that play important roles in embryogenesis, tissue-specific gene expression and tumor development. FOXC1 recognizes and binds to specific DNA sequences through the conserved 110-amino-acid forkhead domain (FH), and thereby activates the target genes. Transactivaton function requires two activation domains, AD-1 and AD-2, and the activity of these domains is attenuated by the inhibitory domain (ID). FOXC1 is suggested to have a key role in cardiac, renal, ocular and cerebral morphogenesis.19
During mouse embryogenesis, both Pitx2 and Foxc1 are expressed in the organs and tissues affected in ARS. In the developing mouse eye, Pitx2 and Foxc1 are co-localized in periocular mesenchyme and they interact physically through crucial functional domains.20 Furthermore, PITX2 can act as a negative regulator of FOXC1 activity, by binding to FOXC1 and repressing activation of putative FOXC1 target genes.20 This interaction has importance in understanding the effects of the different mutations of these genes as explained below.
PITX2 defects and ARS
The most common PITX2 defects leading to ARS are point mutations (Table 2), but several cases of chromosomal aberrations, such as interstitial deletions and/or translocations involving chromosome 4q25, have been described in patients with overlapping ARS phenotypes. Chromosome rearrangements involving PITX2 or its surrounding genomic landscape have been observed in 10 ARS patients,22, 23, 24, 25, 26, 27, 28, 29 and only eight of these cases have been investigated further.23, 24, 25, 26, 28, 29 Three of the cases had deletions at the 4q25 breakpoints directly affecting PITX2,25, 28, 29 and in five cases the translocation breakpoints were located up to 90 kb upstream of the gene.23, 24, 26 In about 14 ARS patients, the genetic defect was a microscopic or submicroscopic deletion of the 4q25 region including PITX2.24, 28, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 In only few cases, the extent of the deletions was characterized by molecular means25, 28, 38 and clinical features of these patients do not differ substantially from the phenotypes caused by point mutations of PITX2.28 Submicroscopic duplications of PITX2 have not been reported in ARS patients, but duplication of the distal region of 4q (including 4q25 and PITX2) has been described in one patient.40
To our knowledge, intragenic mutations of PITX2 have been described in 41 patients and these include, missense (n=18), nonsense (n=4) and splice-site mutations (n=5), and deletions/insertions/duplications (n=14). All these mutations are described in Table 2 and shown in Supplementary Figure 1. Most of the missense mutations (15 out of 18) are within the homeodomain of the gene, which plays a major role in target DNA motif recognition and binding.
PITX2 mutations have also been associated with Peters anomaly,41 iris hypoplasia/iridogoniodysgenesis syndrome,42, 43 and ring dermoid of the cornea,44 but these are single cases and PITX2 mutations are mainly detected in ARS patients with systemic changes.
FOXC1 defects and ARS
The most common FOXC1 defects leading to ARS are point mutations (Table 3), but segmental and telomeric chromosome rearrangements of the chromosome region 6p25, including the FOXC1 gene, occur almost at a similar prevalence.45, 46 Balanced chromosome rearrangements involving FOXC1 or its surrounding landscape are rare, but a balanced t(6;13) translocation in an ARS patient lead to identification of FOXC1, as a causative gene for this disorder.11 On the other hand, there are more than 40 deletions, either interstitial or telomeric, involving 6p2545, 47, 48 and the patients frequently present with ocular, craniofacial, skeletal, cardiac, and renal malformations, hearing loss, and hydrocephalus.47 The phenotypic variation seen in these patients are largely because of the size of the deletions and the genes involved. Ocular anomalies of the anterior segment, such as posterior embryotoxon and iris hypoplasia, are commonly observed in these patients, and these are attributed to the deletion of the FOXC1 gene or its regulatory elements. Furthermore, interstitial duplications of FOXC1 have also been observed in patients with ASD.49, 50, 51
To our knowledge intragenic mutations of FOXC1 have been described in 46 patients and these include missense (n=23) and nonsense mutations (n=6), and deletions/insertions/duplications (n=17). These mutations are described in Table 3 and shown in Supplementary Figure 2. All the missense mutations, except one, are within the forkhead domain and many of these mutations were investigated functionally (Table 3). FOXC1 mutations are mainly detected in ARS patients without extraocular changes, but FOXC1 mutations have also been detected in patients with systemic abnormalities (Table 3). Furthermore, FOXC1 mutations are also detected in rare cases of Peters anomaly,52, 53 iris hypoplasia/iridogoniodysgenesis syndrome,10, 49, 50 primary congenital glaucoma54 and aniridia.55
Disease causing mechanisms of PITX2 and FOXC1 mutations
The mutations observed for PITX2 and FOXC1 show a broad spectrum suggesting that different disease-causing mechanisms lead to ASD.
Both PITX2 and FOXC1 are dosage sensitive and alteration in the level of functional protein (either increased or decreased) is a disease-causative mechanism. Microscopic or submicroscopic deletions of both PITX2 and FOXC1 are known to result in ARS through haploinsufficiency (presence of a single copy of the gene, in which the other copy is inactivated because of mutation). Furthermore, several studies have shown that duplication of FOXC1 may also lead to ARS phenotype. Berry et al20 have shown that FOXC1 transcriptional activity was negatively regulated by PITX2 and this explains how contrasting mutations (duplication of FOXC1 and deletion of PITX2) lead to similar phenotypes: PITX2 binds to FOXC1 and this represses activation of putative FOXC1 target genes. When PITX2 is mutated, the FOXC1 target genes are activated leading to ARS. Duplication of FOXC1 may also have a similar effect, through overcoming the repression activity of PITX2. Identification of target genes regulated by PITX2 and FOXC1 will enable further understanding of the underlying ARS pathologies.
Chromosomal rearrangements some 90 kb upstream of PITX2 may also be pathological and this is likely to be because of removal of some regulatory elements from the gene, leading to the reduction of gene expression.56 Such long-range position effects have previously been suggested for other disorders57 and interestingly FOXC1 has also been associated with long-range position effects.58
Some of the missense mutations leading to amino acid substitutions and other types of mutations have been investigated by functional studies and these mutations alter the protein function in varying degrees (Tables 2–3 and Supplementary Figures 1–2). Most of the intragenic PITX2 mutations are loss-of-function mutations, which result in defective DNA binding, decreased transactivation capability of downstream genes, or both (Table 2). However gain-of-function mutations of PITX2 were also detected (Table 2) and this suggests that excess PITX2 may also lead to ARS. A single mutation with a dominant negative effect on the wild-type protein has also been described (Table 2). Similarly, missense mutations of FOXC1 were shown to effect the nuclear localization, DNA-binding ability and transactivation function of the protein in varying degrees (Table 3).
Phenotypic features of Foxc1 and Pitx2 knockout mice
Homozygous null mutants of Pitx2 generated from a targeted null allele (Pitx2−/−) exhibit septal and valve defects, single atrium, abnormal cardiac positioning, pulmonary isomerism, omphalocele, early arrest in pituitary development, defect in tooth organogenesis, defective development of the mandibular and maxillary facial prominences and multiple eye defects.59, 60, 61 Heterozygote Pitx2+/− mice have thinning of the ventral body, small body size, eye and tooth defects as described in some studies,59, 60 whereas in other studies heterozygote alleles do not show an obvious haplosufficiency phenotype.61 Overexpression of Pitx2 in mouse corneal mesenchyme and iris results in corneal opacification, corneal hypertrophy, irido-corneal adhesions and severely degenerated retina.62
Most homozygous null mutants of Foxc1, generated either from a targeted allele (Foxc1−/−) or the spontaneous mutation congenital hydrocephalus (Foxc1ch/ch) die pre- and perinatally with hemorrhagic hydrocephalus and severe skeletal, cardiovascular and ocular defects.63 The heterozygote Foxc1+/− and Foxc1ch/+ mice have anterior segment abnormalities similar to those observed in patients. These include eccentric and irregularly shaped pupils, iris hypoplasia, displaced Schwalbe's line and abnormal aqueous humor drainage structures, but IOP is not elevated. Interestingly, the penetrance of clinical abnormalities in mice depends on their genetic background, which also would explain the clinical variation observed in ARS patients with similar mutations.64 Even though Foxc1 is suggested to play a critical role in the formation of the atrial septum and cardiac valves during embryogenesis,65 Foxc1+/− mutants do not show cardiovascular abnormalities.66
Genotype–phenotype correlations
Axenfeld–Rieger syndrome is characterized by complete penetrance, but disease severity shows variability. The same mutation may result in different manifestations not only in unrelated patients, but also within the same family. In general, ARS patients who display defects in other organ systems, such as teeth or umbilicus, have mutations of the PITX2 gene; meanwhile, in patients with isolated ASD, mainly FOXC1 mutations are detected.
There is no obvious correlation between the localization of the mutation in the PITX2 gene and the severity of the phenotype (Table 2). For example, the Arg62His mutation within the homeodomain may lead to both ARS and ring dermoid of cornea (Table 2). However, functional studies give clues to the effect of the mutation on the phenotypic outcome (Table 2). An illustrative example is the two consecutive missense mutations within the homeodomain (Val83Leu and Arg84Trp), which lead to ARS and iris hypoplasia, respectively. Functional studies showed that the Val83Leu mutation was a gain-of-function mutation,67 whereas the Arg84Trp mutation resulted in reduced DNA-binding and transactivation,68 giving a plausible explanation to the differences in the clinical severities. The characterized PITX2 missense mutations imply that the severity of the clinical phenotype is proportional to the residual function of the mutant PITX2 proteins.68 In heterozygotes, 50% of the function will result in severe ARS form, but additional activity contributed by mutant allele may result in milder forms.68
FOXC1 mutations are mainly detected in patients with isolated ASD, but systemic abnormalities have also been described (Table 3). The most common extraocular changes observed are heart abnormalities and hearing loss. As for the PITX2 gene, the same FOXC1 mutation may lead to different clinical manifestations. For example, the Leu86Phe mutation leads to ocular changes in one patient; meanwhile, another patient has several systemic abnormalities, including obesity, short stature, myocardial infarction and dental abnormality (Table 3). Functional studies of the FOXC1 missense mutations do not imply a strong correlation between the protein function and the phenotype (Table 3). Strungaru et al69 suggest that patients with FOXC1 duplication have a more severe prognosis in glaucoma development compared to patients with intragenic FOXC1 mutations.
Diagnostic approaches
Clinical diagnosis
In the clinic, ARS patients are mainly diagnosed by the ophthalmologists and the diagnosis remains primarily clinical. As systemic changes are occasional findings of ARS, it will be useful to examine the patient for further changes, such as face and tooth abnormalities (microdontia, hypodontia, oligodontia and adontia), redundant periumbilical skin and facial dysmorphism to facilitate the diagnosis. In case of an ASD, it is important to examine the patient annually with slit lamp examination, including gonioscopy, IOP measurements and funduscopy to assess the retinal nerve fiber layer and optic nerve head, evaluating if the patient develops glaucoma. Autoperimetry (automated measurements of the visual fields) is necessary whenever glaucoma is suspected. As ARS is a congenital disorder, the patient may be very young raising problems in ophthalmological examinations and may necessitate application of general anesthesia.
Genetic diagnosis
A patient with ARS may have a mutation in either of the genes, PITX2 or FOXC1 (Figure 3). Finding of a systemic change is suggestive of a PITX2 mutation, but FOXC1 mutations cannot be excluded from patients with systemic symptoms.
Diagnostic strategy for ARS. A mutation involving PITX2 or FOXC1 can be found in about 40%, leaving the diagnosis in 60% of the patients to rest on clinical features only. Mutation screening of exons 5 and 6 of PITX2; and the first 550 bp of FOXC1 is recommended initially as these regions code for the DNA-binding domains of the respective genes and they are the hot spots for mutations.
As the mutation spectrum for both PITX2 and FOXC1 is very broad, several different methods should be applied for the genetic diagnosis of ARS. The most frequent mutations of PITX2 or FOXC1 are intragenic mutations, which can normally be detected by direct sequencing using patient DNA. Microscopic or submicroscopic deletions (for PITX2 and FOXC1) or duplications (for FOXC1) can be investigated using FISH, quantitative PCR or MLPA (multiplex ligation-dependent probe amplification). High-resolution array-CGH (microarray-based comparative genomic hybridization) may be an advantageous alternative as it is possible to investigate both PITX2 and FOXC1 at the same time with this technology and this may also give the opportunity to isolate a yet unidentified gene associated with ARS. As a substantial number of PITX2 mutations are balanced chromosome rearrangements, classical chromosome analyses should also be carried out in cases, wherein a mutation cannot be detected by the above mentioned methods.
The underlying genetic defect is unknown in ∼60% of ARS patients and partial gene deletions of PITX2 or FOXC1 may be the causative defect in a number of cases. This is illustrated by the finding of an intragenic deletion of PITX2 in an ARS patient using quantitative genomic PCR analysis.70 This deletion can neither be detected by mutation screening because of the presence of the wild-type allele, nor by FISH analysis and array-CGH because of the resolution limit. MLPA may be a suitable method, as it enables to perform multiplex PCR reactions in which several specific sequences are simultaneously quantified. Using this method, it is possible to detect both the submicroscopic chromosome rearrangements and intragenic deletions/duplications of PITX2 and/or FOXC1 simultaneously in ARS patients.
Treatment
The most important symptom that necessitates treatment in ARS is glaucoma. In case of glaucoma, medical therapy is recommended before the initiation of surgical intervention. Medications that decrease aqueous output (β-blockers, α-agonists and carbonic anhydrase inhibitors) are more beneficial than those affecting outflow. However, α-agonists should be used with caution in young children because of the potential for CNS depression.71 If medical therapy is not enough, procedure of choice is trabeculectomy with the adjunctive use of antimetabolites. In case of congenital glaucoma, surgery is preferred. In addition, if photophobia is present in patients with corectopia and polycoria, they may use contact lenses to cover the holes in the iris.
References
Shields MB : Axenfeld–Rieger syndrome: a theory of mechanism and distinctions from the iridocorneal endothelial syndrome. Trans Am Ophthalmol Soc 1983; 81: 736–784.
Alward WLM : Axenfeld–Rieger syndrome in the age of molecular genetics. Am J Ophthalmol 2000; 130: 107–115.
Idrees F, Vaideanu D, Fraser SG, Sowden JC, Khaw PT : A review of anterior segment dysgeneses. Surv Ophthalmol 2006; 51: 213–231.
Sim KT, Krri B, Kaye SB : Posterior embryotoxon may not be a forme fruste of Axenfeld–Rieger's syndrome. J AAPOS 2004; 8: 504–506.
Burian HM, Rice MH, Allen L : External visibility of the Schlemm's canal. Arch Ophthalmol 1957; 57: 651–658.
Townsend WM : Congenital corneal leukomas. Am J Ophthalmol 1974; 77: 80–86.
Tripathy BJ, Tripathy RC : Neural crest origin of human trabecular meshwork and its implications for the pathogenesis of glaucoma. Am J Ophthalmol 1989; 107: 583–590.
Sowden JC : Molecular and developmental mechanisms of anterior segment dysgenesis. Eye 2007; 21: 1310–1318.
Semina EV, Reiter R, Leysens NJ et al: Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet 1996; 14: 392–399.
Mears AJ, Jordan T, Mirzayans F et al: Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld–Rieger anomaly. Am J Hum Genet 1998; 63: 1316–1328.
Nishimura DY, Swiderski RE, Alward WL et al: The forkhead transcription factor gene FKHL7 is responsible for glaucoma phenotypes which map to 6p25. Nat Genet 1998; 19: 140–147.
Stathacopoulos RA, Bateman JB, Sparkes RS et al: The Rieger syndrome and a chromosome 13 deletion. J Pediatr Ophthalmol Strabismus 1987; 24: 198–203.
Phillips JC, del Bono EA, Haines JL et al: A second locus for Rieger syndrome maps to chromosome 13q14. Am J Hum Genet 1996; 59: 613–619.
Werner W, Kraft S, Callen DF, Bartsch O, Hinkel GK : A small deletion of 16q23.1-->16q24.2 [del(16)(q23.1q24.2).ish del(16)(q23.1q24.2)(D16S395+, D16S348-, P5432+)] in a boy with iris coloboma and minor anomalies. Am J Med Genet 1997; 70: 371–376.
Riise R, Storhaug K, Brondum-Nielsen K : Rieger syndrome is associated with PAX6 deletion. Acta Ophthalmol Scand 2001; 79: 201–203.
Hjalt TA, Semina EV : Current molecular understanding of Axenfeld–Rieger syndrome. Expert Rev Mol Med 2005; 7: 1–17.
Amendt BA, Semina EV, Alward WL : Rieger syndrome: a clinical, molecular, and biochemical analysis. Cell Mol Life Sci 2000; 57: 1652–1666.
Cox CJ, Espinoza HM, McWilliams B et al: Differential regulation of gene expression by PITX2 isoforms. J Biol Chem 2002; 277: 25001–25010.
Lehmann OJ, Sowden JC, Carlsson P, Jordan T, Bhattacharya SS : Fox's in development and disease. Trends Genet 2003; 19: 339–344.
Berry FB, Lines MA, Oas JM et al: Functional interactions between FOXC1 and PITX2 underlie the sensitivity to FOXC1 gene dose in Axenfeld–Rieger syndrome and anterior segment dysgenesis. Hum Mol Genet 2006; 15: 905–919.
Den Dunnen JT, Antonorakis SE : Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat 2000; 15: 7–12.
Makita Y, Masuno M, Imaizumi K et al: Rieger syndrome with de novo reciprocal translocation t(1; 4)(q23.1D5). Am J Med Genet 1995; 57: 19–21.
Datson NA, Semina E, van Staalduinen AA et al: Closing in on the Rieger syndrome gene on 4q25: mapping translocation breakpoints within a 50-kb region. Am J Hum Genet 1996; 59: 1297–1305.
Flomen RH, Gorman PA, Vatcheva R et al: Rieger syndrome locus: a new reciprocal translocation t(4;12)(q25;q15) and a deletion del(4)(q25q27) both break between markers D4S2945 and D4S193. J Med Genet 1997; 34: 191–195.
Kamnasaran D, O'Brien PC, Zackai EH et al: Rearrangement in the PITX2 and MIPOL1 genes in a patient with a t(4;14) chromosome. Eur J Hum Genet 2003; 11: 315–324.
Trembath DG, Semina EV, Jones DH et al: Analysis of two translocation breakpoints and identification of a negative regulatory element in patients with Rieger's syndrome. Birth Defects Res A Clin Mol Teratol 2004; 70: 82–91.
Karadeniz NN, Kocak-Midillioglu I, Erdogan D et al: Is SHORT syndrome another phenotypic variation of PITX2? Am J Med Genet A 2004; 130: 406–409.
Engenheiro E, Saraiva J, Carreira I et al: Cytogenetically invisible microdeletions involving PITX2 in Rieger syndrome. Clin Genet 2007; 72: 464–470.
Mihelec M, St Heaps L, Flaherty M et al: Chromosomal rearrangements and novel genes in disorders of eye development, cataract and glaucoma. Twin Res Hum Genet 2008; 11: 412–421.
Serville F, Broustet A : Pericentric inversion and partial monosomy 4q associated with congenital anomalies. Hum Genet 1977; 39: 239–242.
Mitchell JA, Packman S, Loughman WD et al: Deletions of different segments of the long arm of chromosome 4. Am J Med Genet 1981; 8: 73–89.
Chudley AE, Verna MR, Ray M et al: Interstitial deletion of the long arm of chromosome 4. Am J Med Genet 1988; 31: 549–551.
Raczenbek C, Krassikoff N, Cosper P : Second case report of del(4) (q25q27) and review of the literature. Clin Genet 1991; 39: 463–466.
Fryns JP, van den Berghe H : Rieger syndrome and interstitial 4q26 deletion. Genet Couns 1992; 3: 153–154.
Vaux C, Sheffield L, Keith CG, Voullaire L : Evidence that Rieger syndrome maps to 4q25 or 4q27. J Med Genet 1992; 29: 256–258.
Kulharya AS, Maberry M, Kukolich MK et al: Interstitial deletions 4q21.1q25 and 4q25q27: phenotypic variability and relation to Rieger anomaly. Am J Med Genet 1995; 55: 165–170.
Schinzel A, Brecevic L, Dutly F, Baumer A, Binkert F, Largo RH : Multiple congenital anomalies including the Rieger eye malformation in a boy with interstitial deletion of (4) (q25-->q27) secondary to a balanced insertion in his normal father: evidence for haplotype insufficiency causing the Rieger malformation. J Med Genet 1997; 34: 1012–1014.
Lines MA, Kozlowski K, Walter MA : Molecular genetics of Axenfeld–Rieger malformations. Hum Mol Genet 2002; 11: 1177–1184.
Becker SA, Popp S, Rager K, Jauch A : A new case of an interstitial deletion (4)(q25q27) characterised by molecular cytogenetic techniques and review of the literature. Eur J Pediatr 2003; 162: 267–270.
Velinov M, Gu H, Yeboa K et al: Hypoplastic left heart in a female infant with partial trisomy 4q due to de novo 4;21 translocation. Am J Med Genet 2002; 107: 330–333.
Doward W, Perveen R, Lloyd IC, Ridgway AE, Wilson L, Black GC : A mutation in the RIEG1 gene associated with Peters' anomaly. J Med Genet 1999; 36: 152–155.
Alward WL, Semina EV, Kalenak JW et al: Autosomal dominant iris hypoplasia is caused by a mutation in the Rieger syndrome (RIEG/PITX2) gene. Am J Ophthalmol 1998; 125: 98–100.
Kulak SC, Kozlowski K, Semina EV, Pearce WG, Walter MA : Mutation in the RIEG1 gene in patients with iridogoniodysgenesis syndrome. Hum Mol Genet 1998; 7: 1113–1117.
Xia K, Wu L, Liu X et al: Mutation in PITX2 is associated with ring dermoid of the cornea. J Med Genet 2004; 41: e129.
Gould DB, Jaafar MS, Addison MK et al: Phenotypic and molecular assessment of seven patients with 6p25 deletion syndrome: relevance to ocular dysgenesis and hearing impairment. BMC Med Genet 2004; 5: 17.
Chanda B, Asai-Coakwell M, Ye M et al: A novel mechanistic spectrum underlies glaucoma associated chromosome 6p25 copy number variation. Hum Mol Genet 2008; 17: 3446–3458, Aug 11 (E-pub ahead of print).
Le Caignec C, De Mas P, Vincent MC et al: Subtelomeric 6p deletion: clinical, FISH, and array CGH characterization of two cases. Am J Med Genet A 2005; 132A: 175–180.
Descipio C, Schneider L, Young TL et al: Subtelomeric deletions of chromosome 6p: molecular and cytogenetic characterization of three new cases with phenotypic overlap with Ritscher–Schinzel (3C) syndrome. Am J Med Genet A 2005; 134A: 3–11.
Lehmann OJ, Ebenezer ND, Jordan T et al: Chromosomal duplication involving the forkhead transcription factor gene FOXC1 causes iris hypoplasia and glaucoma. Am J Hum Genet 2000; 67: 1129–1135.
Nishimura DY, Searby CC, Alward WL et al: A spectrum of FOXC1 mutations suggests gene dosage as a mechanism for developmental defects of the anterior chamber of the eye. Am J Hum Genet 2001; 68: 364–372.
Lehmann OJ, Ebenezer ND, Ekong R et al: Ocular developmental abnormalities and glaucoma associated with interstitial 6p25 duplications and deletions. Invest Ophthalmol Vis Sci 2002; 43: 1843–1849.
Honkanen RA, Nishimura DY, Swiderski RE et al: A family with Axenfeld–Rieger syndrome and Peters anomaly caused by a point mutation (Phe112Ser) in the FOXC1 gene. Am J Ophthalmol 2003; 135: 368–375.
Weisschuh N, Wolf C, Wissinger B, Gramer E : A novel mutation in the FOXC1 gene in a family with Axenfeld–Rieger syndrome and Peters' anomaly. Clin Genet 2008; 74: 476–480, May 21(E-pub ahead of print).
Chakrabarti S, Kaur K, Rao KN et al: The transcirption factor gene FOXC1 exhibits a limited role in primary congenital glaucoma. Invest Ophthal Vis Sci 2009; 50: 75–83.
Khan A, Aldahmesh MA, Al-Amri A : Heterozygous FOXC1 mutation (M161K) associated with congenital glaucoma and aniridia in an infant and a milder phenotype in her mother. Ophthalmic Genet 2008; 29: 67–71.
Flomen RH, Vatcheva R, Gorman PA et al: Construction and analysis of a sequence-ready map in 4q25: Rieger syndrome can be caused by haploinsufficiency of RIEG, but also by chromosome breaks approximately 90 kb upstream of this gene. Genomics 1998; 47: 409–413.
Kleinjan DA, Lettice LA : Chapter 13 long-range gene control and genetic disease. Adv Genet 2008; 61: 339–388.
Davies AF, Mirza G, Sekhon G et al: Delineation of two distinct 6p deletion syndromes. Hum Genet 1999; 104: 64–72.
Gage PJ, Suh H, Camper SA : Dosage requirement of Pitx2 for development of multiple organs. Development 1999; 126: 4643–4651.
Lin CR, Kioussi C, O'Connell S et al: Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature 1999; 401: 279–282.
Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF : Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature 1999; 401: 276–278.
Holmberg J, Liu CY, Hjalt TA : PITX2 gain-of-function in Rieger syndrome eye model. Am J Pathol 2004; 165: 1633–1641.
Kume T, Deng KY, Winfrey V, Gould DB, Walter MA, Hogan BL : The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell 1998; 93: 985–996.
Smith RS, Zabaleta A, Kume T et al: Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum Mol Genet 2000; 9: 1021–1032.
Swiderski RE, Reiter RS, Nishimura DY et al: Expression of the Mf1 gene in developing mouse hearts: implication in the development of human congenital heart defects. Dev Dyn 1999; 216: 16–27.
Winnier GE, Kume T, Deng K et al: Roles for the winged helix transcription factors MF1 and MFH1 in cardiovascular development revealed by nonallelic noncomplementation of null alleles. Dev Biol 1999; 213: 418–431.
Priston M, Kozlowski K, Gill D et al: Functional analyses of two newly identified PITX2 mutants reveal a novel molecular mechanism for Axenfeld–Rieger syndrome. Hum Mol Genet 2001; 10: 1631–1638.
Kozlowski K, Walter MA : Variation in residual PITX2 activity underlies the phenotypic spectrum of anterior segment developmental disorders. Hum Mol Genet 2000; 9: 2131–2139.
Strungaru MH, Dinu I, Walter MA : Genotype-phenotype correlations in Axenfeld–Rieger malformation and glaucoma patients with FOXC1 and PITX2 mutations. Invest Ophthalmol Vis Sci 2007; 48: 228–237.
de la Houssaye G, Bieche I, Roche O et al: Identification of the first intragenic deletion of the PITX2 gene causing an Axenfeld–Rieger syndrome: case report. BMC Med Genet 2006; 7: 82.
Enyedi LB, Freedman SF : Safety and efficacy of brimonidine in children with glaucoma. J AAPOS 2001; 5: 281–284.
Further reading
Alward WLM : Axenfeld–Rieger syndrome in the Age of Molecular Genetics. Am J Ophthalmol 2000; 130: 107–115.
Amendt BA, Semina EV, Alward WL : Rieger syndrome: a clinical, molecular, and biochemical analysis. Cell Mol Life Sci 2000; 57: 1652–1666.
Hjalt TA, Semina EV : Current molecular understanding of Axenfeld–Rieger syndrome. Expert Rev Mol Med 2005; 7: 1–17.
Idrees F, Vaideanu D, Fraser SG, Sowden JC, Khaw PT : A review of anterior segment dysgeneses. Survey of Ophthalmol 2006; 51: 213–231.
Sowden JC : Molecular and developmental mechanisms of anterior segment dysgenesis. Eye 2007; 21: 1310–1318.
Acknowledgements
We thank Drs Hanne Jensen, John Thygesen and Karen Brøndum Nielsen for their comments on the paper. We also acknowledge The Glaucoma Clinic, Rigshospitalet, Copenhagen, Denmark and Professor Mette Warburg for kindly providing the clinical photographs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Rights and permissions
About this article
Cite this article
Tümer, Z., Bach-Holm, D. Axenfeld–Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur J Hum Genet 17, 1527–1539 (2009). https://doi.org/10.1038/ejhg.2009.93
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2009.93
This article is cited by
-
Diagnostik, Klinik und Genetik kongenitaler Hornhauttrübungen
Der Ophthalmologe (2022)
-
A novel variant in FOXC1 associated with atypical Axenfeld-Rieger syndrome
BMC Medical Genomics (2021)
-
A missense mutation in Pitx2 leads to early-onset glaucoma via NRF2-YAP1 axis
Cell Death & Disease (2021)
-
DNA-based eyelid trait prediction in Chinese Han population
International Journal of Legal Medicine (2021)
-
Expression of smooth muscle-like effectors and core cardiomyocyte regulators in the contractile papillae of Ciona
EvoDevo (2020)