Abstract
Background/Objectives:
Easy-to-use and accurate methods to assess free-living activity energy expenditure (AEE) in preschool children are required. The aims of this study in healthy preschool children were to (a) evaluate the ability of the wrist-worn ActiGraph wGT3x-BT to predict free-living AEE and (b) assess wear compliance using a 7-day, 24-h protocol.
Subjects/Methods:
Participants were 40 Swedish children (5.5±0.2 years) in the Mobile-based intervention intended to stop obesity in preschoolers (MINISTOP) obesity prevention trial. Total energy expenditure (TEE) was assessed using the doubly labeled water method during 14 days. AEE was calculated as (TEEx0.9) minus predicted basal metabolic rate. The ActiGraph accelerometer was worn on the wrist for 7 days and outputs used were mean of the daily and awake filtered vector magnitude (mean VM total and mean VM waking).
Results:
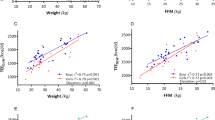
The ActiGraph was worn for 7 (n=34, 85%), 6 (n=4, 10%), 5 (n=1, 2.5%) and 4 (n=1, 2.5%) days (a valid day was ⩾600 awake minutes). Alone, mean VM total and mean VM waking were able to explain 14% (P=0.009) and 24% (P=0.001) of the variation in AEE, respectively. By incorporating fat and fat-free mass in the models 58% (mean VM total) and 62% (mean VM waking) in the variation of AEE was explained (P<0.001).
Conclusions:
The wrist-worn ActiGraph wGT3x-BT in combination with body composition variables explained up to the 62% of the variation in AEE. Given the high wear compliance, the wrist-worn ActiGraph has the potential to provide useful information in studies where physical activity in preschool children is measured.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
World Health Organization. Global Recommendations on Physical Activity for Health. Global Strategy on Diet, Physical Activity and Health. World Health Organization: Geneva, Switzerland, 2010.
Tremblay MS, Aguilar-Farias N, Akinroye KK, Al-Kuwari MG, Amornsriwatanakul A, Aubert S et al. Global Matrix 2.0: report card grades on the physical activity of children and youth comparing 38 countries. J Phys Act Health 2016; 13 (Suppl 2), S343–S366.
Hills AP, Okely AD, Baur LA . Addressing childhood obesity through increased physical activity. Nat Rev Endocrinol 2010; 6: 543–549.
World Health Organization Report of the Commission on Ending Childhood Obesity. Global Strategy on Diet, Physical Activity and Health. World Health Organization: Geneva, Switzerland, 2016.
International Atomic Energy Agency Assessment of Body Composition and Total Energy Expenditure in Humans Using Stable Isotope Techniques, IAEA Human Health Series No. 3. International Atomic Energy Agency: Vienna, Austria, 2009.
Butte NF, Ekelund U, Westerterp KR . Assessing physical activity using wearable monitors: measures of physical activity. Med Sci Sports Exerc 2012; 44 (Suppl 1), S5–S12.
Jeran SSA, Pischon T . Prediction of activity related energy expenditure using accelerometer derived physical activity under free-living conditions—a systematic review. Int J Obes 2016; 40: 1187–1197.
Fairclough SJ, Noonan R, Rowlands AV, van Hees V, Knowles Z, Boddy LM . Wear compliance and activity in children wearing wrist and hip-mounted accelerometers. Med Sci Sports Exerc 2016; 48: 245–253.
Freedson PS, John D . Comment on 'estimating activity and sedentary behavior from an accelerometer on the hip and wrist'. Med Sci Sports Exerc 2013; 45: 962–963.
van Hees VT, Renstrom F, Wright A, Gradmark A, Catt M, Chen KY et al. Estimation of daily energy expenditure in pregnant and non-pregnant women using a wrist-worn tri-axial accelerometer. PLoS One 2011; 6: e22922.
Troiano RP, McClain JJ, Brychta RJ, Chen KY . Evolution of accelerometer methods for physical activity research. Br J Sports Med 2014; 48: 1019–1023.
Lopez-Alarcon M, Merrifield J, Fields DA, Hilario-Hailey T, Franklin FA, Shewchuk RM et al. Ability of the actiwatch accelerometer to predict free-living energy expenditure in young children. Obesity Res 2004; 12: 1859–1865.
Sijtsma A, Schierbeek H, Goris AH, Joosten KF, van Kessel I, Corpeleijn E et al. Validation of the TracmorD triaxial accelerometer to assess physical activity in preschool children. Obesity 2013; 21: 1877–1883.
Henriksson H, Forsum E, Lof M . Evaluation of Actiheart and a 7 d activity diary for estimating free-living total and activity energy expenditure using criterion methods in 1.5- and 3-year-old children. Br J Nutr 2014; 111: 1830–1840.
Montgomery C, Reilly JJ, Jackson DM, Kelly LA, Slater C, Paton JY et al. Relation between physical activity and energy expenditure in a representative sample of young children. Am J Clin Nutr 2004; 80: 591–596.
Butte NF, Wong WW, Lee JS, Adolph AL, Puyau MR, Zakeri IF . Prediction of energy expenditure and physical activity in preschoolers. Med Sci Sports Exerc 2014; 46: 1216–1226.
Ojiambo R, Konstabel K, Veidebaum T, Reilly J, Verbestel V, Huybrechts I et al. Validity of hip-mounted uniaxial accelerometry with heart-rate monitoring vs. triaxial accelerometry in the assessment of free-living energy expenditure in young children: the IDEFICS Validation Study. J Appl Physiol 2012; 113: 1530–1536.
Reilly JJ, Kelly LA, Montgomery C, Jackson DM, Slater C, Grant S et al. Validation of Actigraph accelerometer estimates of total energy expenditure in young children. Int J Pediatr Obes 2006; 1: 161–167.
Delisle C, Sandin S, Forsum E, Henriksson H, Trolle-Lagerros Y, Larsson C et al. A web- and mobile phone-based intervention to prevent obesity in 4-year-olds (MINISTOP): a population-based randomized controlled trial. BMC Public Health 2015; 15: 95.
Delisle Nystrom C, Sandin S, Henriksson P, Henriksson H, Trolle Lagerros Y, Larsson C et al. Mobile-based intervention intended to stop obesity in pre-school children: the MINISTOP randomized controlled trial. Am J Clin Nutr 2017; 105: 1327–1335.
Leppanen MH, Nystrom CD, Henriksson P, Pomeroy J, Ruiz JR, Ortega FB et al. Physical activity intensity, sedentary behavior, body composition and physical fitness in 4-year-old children: results from the MINISTOP trial. Int J Obes 2016; 40: 1126–1133.
Delisle Nystrom C, Forsum E, Henriksson H, Trolle-Lagerros Y, Larsson C, Maddison R et al. A mobile phone based method to assess energy and food intake in young children: a validation study against the doubly labelled water method and 24h dietary recalls. Nutrients 2016; 8 (1). doi:10.3390/nu8010050.
Davies PS, Coward WA, Gregory J, White A, Mills A . Total energy expenditure and energy intake in the pre-school child: a comparison. Br J Nutr 1994; 72: 13–20.
Weir J . New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949; 109: 1–9.
Black A, Prentice A, Coward W . Use of food quotients to predict respiratory quotients for the doubly-labelled water method of measuring energy expenditure. Hum Nutr Clin Nutr 1986; 40: 381–391.
Wells JC, Williams JE, Chomtho S, Darch T, Grijalva-Eternod C, Kennedy K et al. Pediatric reference data for lean tissue properties: density and hydration from age 5 to 20 y. Am J Clin Nutr 2010; 91: 610–618.
The Nordic Council of Ministers Nordic Nutrient Recommendations 2012, Integrating Nutrition and Physical Activity, 5th edn. The Nordic Council of Ministers: Copenhagen, Denmark, 2012.
Bouten CV, Koekkoek KT, Verduin M, Kodde R, Janssen JD . A triaxial accelerometer and portable data processing unit for the assessment of daily physical activity. IEEE Trans Biomed Eng 1997; 44: 136–147.
Sadeh A, Lavie P, Scher A, Tirosh E, Epstein R . Actigraphic home-monitoring sleep-disturbed and control infants and young children: a new method for pediatric assessment of sleep–wake patterns. Pediatrics 1991; 87: 494–499.
Sadeh A, Sharkey KM, Carskadon MA . Activity-based sleep–wake identification: an empirical test of methodological issues. Sleep 1994; 17: 201–207.
Collings PJ, Brage S, Ridgway CL, Harvey NC, Godfrey KM, Inskip HM et al. Physical activity intensity, sedentary time, and body composition in preschoolers. Am J Clin Nutr 2013; 97: 1020–1028.
Armitage P, Berry G, Mathews JNS . Statistical Methods in Medical Research. Blackwell Science: Oxford, UK, 2002.
Assah FK, Ekelund U, Brage S, Corder K, Wright A, Mbanya JC et al. Predicting physical activity energy expenditure using accelerometry in adults from sub-Sahara Africa. Obesity 2009; 17: 1588–1595.
Bland JM, Altman DG . Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310.
Kleinbaum DG KL, Nizam A, Muller KE . Applied Regression Analysis and Other Multivariable Methods, 4th edn. Thomson: Belmont, CA, USA, 2008.
Rosenberger ME, Haskell WL, Albinali F, Mota S, Nawyn J, Intille S . Estimating activity and sedentary behavior from an accelerometer on the hip or wrist. Med Sci Sports Exerc 2013; 45: 964–975.
Bonomi AG, Plasqui G, Goris AH, Westerterp KR . Estimation of free-living energy expenditure using a novel activity monitor designed to minimize obtrusiveness. Obesity 2010; 18: 1845–1851.
Masse LC, Fulton JE, Watson KL, Mahar MT, Meyers MC, Wong WW . Influence of body composition on physical activity validation studies using doubly labeled water. J Appl Physiol 2004; 96: 1357–1364.
Corder K, Brage S, Wright A, Ramachandran A, Snehalatha C, Yamuna A et al. Physical activity energy expenditure of adolescents in India. Obesity 2010; 18: 2212–2219.
Coward WA, Cole TJ. The double labeled water method for the measurement of energy expenditure in humans: risks and benefits. In: Whitehead RG, Prentice A (eds). New Techniques in Nutritional Research, 9th edn. Academic Press: San Diego, CA, USA, 1991; pp 139–176.
Speakman JR . Double Labelled Water. Theory and Practice, 1st edn. Chapman & Hall: London, UK, 1997.
Public Health Agency of SwedenOverweight and Obesity National Statistics, 2014. Available at: http://www.folkhalsomyndigheten.se/ (last accessed September 2016).
Bornhorst C, Bel-Serrat S, Pigeot I, Huybrechts I, Ottavaere C, Sioen I et al. Validity of 24-h recalls in (pre-)school aged children: comparison of proxy-reported energy intakes with measured energy expenditure. Clin Nutr 2014; 33: 79–84.
Wikland KA, Luo ZC, Niklasson A, Karlberg J . Swedish population-based longitudinal reference values from birth to 18 years of age for height, weight and head circumference. Acta Paediatr 2002; 91: 739–754.
Statistics Sweden. Educational Attainment of the Population, 2015. Available at: http://www.scb.se/ (last accessed September 2016).
Fomon SJ, Haschke F, Ziegler EE, Nelson SE . Body composition of reference children from birth to age 10 years. Am J Clin Nutr 1982; 35 (Suppl), 1169–1175.
Roberts SB, Young VR . Energy costs of fat and protein deposition in the human infant. Am J Clin Nutr 1988; 48: 951–995.
Cole TJ, Lobstein T . Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes 2012; 7: 284–294.
Acknowledgements
CDN was supported by the SNF Swedish Nutrition Foundation; ML by the Swedish Research Council (project no. 2012–2883), the Swedish Research Council for Health, Working Life and Welfare (2012-0906), Bo and Vera Axson Johnsons Foundation and Karolinska Institutet; and PH by Henning and Johan Throne-Holst Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Delisle Nyström, C., Pomeroy, J., Henriksson, P. et al. Evaluation of the wrist-worn ActiGraph wGT3x-BT for estimating activity energy expenditure in preschool children. Eur J Clin Nutr 71, 1212–1217 (2017). https://doi.org/10.1038/ejcn.2017.114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2017.114
This article is cited by
-
Impact of ambient air pollution on physical activity and sedentary behavior in children
BMC Public Health (2023)
-
A systematic review of the validity, reliability, and feasibility of measurement tools used to assess the physical activity and sedentary behaviour of pre-school aged children
International Journal of Behavioral Nutrition and Physical Activity (2021)
-
A 12-month follow-up of a mobile-based (mHealth) obesity prevention intervention in pre-school children: the MINISTOP randomized controlled trial
BMC Public Health (2018)