Abstract
Background/Objectives:
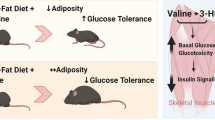
Branched chain amino acids (BCAA) are among nutrients strongly linked with insulin sensitivity (IS) measures. We investigated the effects of a chronic increase of BCAA intake on IS in two groups of healthy subjects differing in their basal consumption of BCAA, that is, vegans and omnivores.
Subjects/Methods:
Eight vegans and eight matched omnivores (five men and three women in each group) received 15 g (women) or 20 g (men) of BCAA daily for 3 months. Anthropometry, blood analyses, glucose clamp, arginine test, subcutaneous abdominal adipose tissue (AT) and skeletal muscle (SM) biopsies (mRNA levels of selected metabolic markers, respiratory chain (RC) activity) were performed at baseline, after the intervention and after a 6 month wash-out period.
Results:
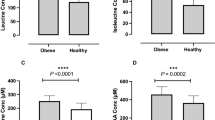
Compared with omnivores, vegans had higher IS at baseline (GIR, glucose infusion rate: 9.6±2.4 vs 7.1±2.4 mg/kg/min, 95% CI for difference: 0.55 to 5.82) that declined after the intervention and returned to baseline values after the wash-out period (changes in GIR with 95% CI, 3–0 months: −1.64 [−2.5; −0.75] and 9-3 months: 1.65 [0.75; 2.54] mg/kg/min). No such change was observed in omnivores. In omnivores the intervention led to an increased expression of lipogenic genes (DGAT2, FASN, PPARγ, SCD1) in AT. SM RC activity increased in both groups.
Conclusions:
Negative impact of increased BCAA intake on IS was only detected in vegans, that is, subjects with low basal amino acids/BCAA intake, which appear to be unable to induce sufficient compensatory changes within AT and SM on a BCAA challenge.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009; 9: 311–326.
Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB . Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem 2010; 285: 11348–11356.
Pedroso JAB, Zampieri TT, Donato J . Reviewing the effects of l-Leucine supplementation in the regulation of food intake, energy balance, and glucose homeostasis. Nutrients 2015; 7: 3914–3937.
Green CR, Wallace M, Divakaruni AS, Phillips SA, Murphy AN, Ciaraldi TP et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol 2016; 12: 15–21.
Felig P, Marliss E, Cahill GF J . Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 1969; 281: 811–816.
Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011; 17: 448–453.
Everman S, Mandarino LJ, Carroll CC, Katsanos CS . Effects of acute exposure to increased plasma branched-chain amino acid concentrations on insulin-mediated plasma glucose turnover in healthy young subjects. PLoS One 2015; 10: e0120049.
Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 2005; 54: 2674–2684.
Robinson MM, Soop M, Sohn TS, Morse DM, Schimke JM, Klaus KA et al. High insulin combined with essential amino acids stimulates skeletal muscle mitochondrial protein synthesis while decreasing insulin sensitivity in healthy humans. J Clin Endocrinol Metab 2014; 99: E2574–E2583.
Clarys P, Deliens T, Huybrechts I, Deriemaeker P, Vanaelst B, De Keyzer W et al. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014; 6: 1318–1332.
Souci SW, Fachmann W, Kraut H. Food composition and nutrition tables. 2000 http://www.cabdirect.org/abstracts/20001418574.html;jsessionid=46277F47F16A2E0B8F137D24E5143C20 (accessed 20 January 2016).
Gojda J, Patková J, Jaček M, Potočková J, Trnka J, Kraml P et al. Higher insulin sensitivity in vegans is not associated with higher mitochondrial density. Eur J Clin Nutr 2013; 67: 1–6.
Baecke JA, Burema J, Frijters JE . A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982; 36: 936–942.
Tůma P, Gojda J . Rapid determination of branched chain amino acids in human blood plasma by pressure-assisted capillary electrophoresis with contactless conductivity detection. Electrophoresis 2015; 36: 1969–1975.
DeFronzo RA, Tobin JD, Andres R . Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214–E223.
Palmer JP, Benson JW, Walter RM, Ensinck JW . Arginine-stimulated acute phase of insulin and glucagon secretion in diabetic subjects. J Clin Invest 1976; 58: 565–570.
Bergstrom J . Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 1975; 35: 609–616.
Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C . Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc 2012; 7: 1235–1246.
van Loon LJ, Saris WH, Verhagen H, Wagenmakers AJ . Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am J Clin Nutr 2000; 72: 96–105.
Nair KS, Schwartz RG, Welle S . Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol 1992; 263: E928–E934.
Takeshita Y, Takamura T, Kita Y, Ando H, Ueda T, Kato K et al. Beneficial effect of branched-chain amino acid supplementation on glycemic control in chronic hepatitis C patients with insulin resistance: implications for type 2 diabetes. Metabolism 2012; 61: 1388–1394.
Macotela Y, Emanuelli B, Bång AM, Espinoza DO, Boucher J, Beebe K et al. Dietary leucine–an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One 2011; 6: e21187–e21187.
Ouellet V, Marois J, Weisnagel SJ, Jacques H . Dietary cod protein improves insulin sensitivity in insulin-resistant men and women: a randomized controlled trial. Diabetes Care 2007; 30: 2816–2821.
Jakubowicz D, Froy O, Ahrén B, Boaz M, Landau Z, Bar-Dayan Y et al. Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: a randomised clinical trial. Diabetologia 2014; 57: 1807–1811.
Yang J, Chi Y, Burkhardt BR, Guan Y, Wolf BA . Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev 2010; 68: 270–279.
Ueno M, Carvalheira JBC, Tambascia RC, Bezerra RMN, Amaral ME, Carneiro EM et al. Regulation of insulin signalling by hyperinsulinaemia: role of IRS-1/2 serine phosphorylation and the mTOR/p70 S6K pathway. Diabetologia 2005; 48: 506–518.
Tumova J, Andel M, Trnka J . Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol Res 2015; 65: 193–207.
Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab 2013; 304: E1175–E1187.
Obata A, Kubota N, Kubota T, Iwamoto M, Sato H, Sakurai Y et al. Tofogliflozin improves insulin resistance in skeletal muscle and accelerates lipolysis in adipose tissue in male mice. Endocrinology 2016; 157: 1029–1042.
Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes 2007; 56: 1600–1607.
Pawlak R, Lester SE, Babatunde T . The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: a review of literature. Eur J Clin Nutr 2014; 68: 541–548.
Acknowledgements
This study was funded within the scientific framework of research programs of Charles University in Prague, PRVOUK-P31 and UNCE 204015, and was supported by a Grant of the Ministry of Health of the Czech Republic, number NT/14416.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gojda, J., Rossmeislová, L., Straková, R. et al. Chronic dietary exposure to branched chain amino acids impairs glucose disposal in vegans but not in omnivores. Eur J Clin Nutr 71, 594–601 (2017). https://doi.org/10.1038/ejcn.2016.274
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2016.274
This article is cited by
-
Dietary intake of branched-chain amino acids in relation to depression, anxiety and psychological distress
Nutrition Journal (2021)
-
Higher intakes of energy-adjusted dietary amino acids are inversely associated with obesity risk
Amino Acids (2019)
-
The use of coupled gas chromatography columns for the determination of individual isomers of trans fatty acids in the adipose tissue of vegans
Monatshefte für Chemie - Chemical Monthly (2019)