Abstract
Background/Objectives:
Dietary pattern analysis considers combinations of food intake and may offer a better measure to assess diet–cancer associations than examining individual foods or nutrients. Although tobacco exposure is the major risk factor for lung cancer, few studies have examined whether dietary patterns, based on preexisting dietary guidelines, influence lung cancer risk. After controlling for smoking, we examined associations between four diet quality indices—Healthy Eating Index–2010 (HEI-2010), Alternate Healthy Eating Index–2010 (AHEI-2010), alternate Mediterranean Diet score (aMED) and Dietary Approaches to Stop Hypertension (DASH)—and lung cancer risk in the NIH–AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health study.
Subjects/Methods:
Baseline dietary intake was assessed in 460 770 participants. Over a median of 10.5 years of follow-up, 9272 incident lung cancer cases occurred. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and confidence intervals (CIs).
Results:
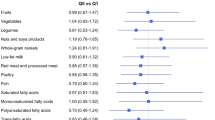
Comparing highest to lowest quintiles, HRs (95% CIs) for lung cancer were as follows: HEI-2010=0.83 (0.77–0.89), AHEI-2010=0.86 (0.80–0.92), aMED=0.85 (0.79–0.91) and DASH=0.84 (0.78–0.90). Among the individual components of the dietary indices, higher consumption of whole grains and fruits was significantly inversely associated with lung cancer risk for several of the diet indices. Total index score analyses stratified by smoking status showed inverse associations with lung cancer for former smokers; however, only HEI-2010 was inversely associated in current smokers and no index score was inversely associated among never smokers.
Conclusions:
Although smoking is the factor most strongly associated with lung cancer, this study adds to a growing body of evidence that diet may have a modest role in reducing lung cancer risk, especially among former smokers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
American Cancer Society Cancer Facts and Figures http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2013, 2013.
World Cancer Research Fund/American Institute for Cancer Research Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. AICR: Washington, DC, 2007.
Lam TK, Gallicchio L, Lindsley K, Shiels M, Hammond E, Tao XG et al. Cruciferous vegetable consumption and lung cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev 2009; 18: 184–195.
Arts IC . A review of the epidemiological evidence on tea, flavonoids, and lung cancer. J Nutr 2008; 138: 1561S–1566S.
Chao C . Associations between beer, wine, and liquor consumption and lung cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2007; 16: 2436–2447.
Smith-Warner SA, Spiegelman D, Yaun SS, Albanes D, Beeson WL, van den Brandt PA et al. Fruits, vegetables and lung cancer: a pooled analysis of cohort studies. Int J Cancer 2003; 107: 1001–1011.
Daniel CR, Cross AJ, Graubard BI, Hollenbeck AR, Park Y, Sinha R . Prospective investigation of poultry and fish intake in relation to cancer risk. Cancer Prev Res (Phila) 2011; 4: 1903–1911.
Tasevska N, Sinha R, Kipnis V, Subar AF, Leitzmann MF, Hollenbeck AR et al. A prospective study of meat, cooking methods, meat mutagens, heme iron, and lung cancer risks. Am J Clin Nutr 2009; 89: 1884–1894.
Wright ME, Park Y, Subar AF, Freedman ND, Albanes D, Hollenbeck A et al. Intakes of fruit, vegetables, and specific botanical groups in relation to lung cancer risk in the NIH-AARP Diet and Health Study. Am J Epidemiol 2008; 168: 1024–1034.
Hu FB . Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002; 13: 3–9.
Moeller SM, Reedy J, Millen AE, Dixon LB, Newby PK, Tucker KL et al. Dietary patterns: challenges and opportunities in dietary patterns research an Experimental Biology workshop, April 1, 2006.. J Am Diet Assoc 2007; 107: 1233–1239.
Gnagnarella P, Maisonneuve P, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L et al. Red meat, Mediterranean diet and lung cancer risk among heavy smokers in the COSMOS screening study. Ann Oncol 2013; 24: 2606–2611.
Gnagnarella P, Maisonneuve P, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L et al. Nutrient intake and nutrient patterns and risk of lung cancer among heavy smokers: results from the COSMOS screening study with annual low-dose CT. Eur J Epidemiol 2013; 28: 503–511.
Gorlova OY, Weng SF, Hernandez L, Spitz MR, Forman MR . Dietary patterns affect lung cancer risk in never smokers. Nutr Cancer 2011; 63: 842–849.
De Stefani E, Ronco AL, Deneo-Pellegrini H, Correa P, Boffetta P, Acosta G et al. Dietary patterns and risk of adenocarcinoma of the lung in males: a factor analysis in Uruguay. Nutr Cancer 2011; 63: 699–706.
De Stefani E, Deneo-Pellegrini H, Boffetta P, Ronco AL, Aune D, Acosta G et al. Dietary patterns and risk of cancer: a factor analysis in Uruguay. Int J Cancer 2009; 124: 1391–1397.
De Stefani E, Boffetta P, Ronco AL, Deneo-Pellegrini H, Acosta G, Gutierrez LP et al. Nutrient patterns and risk of lung cancer: a factor analysis in Uruguayan men. Lung Cancer 2008; 61: 283–291.
Balder HF, Goldbohm RA, van den Brandt PA . Dietary patterns associated with male lung cancer risk in the Netherlands Cohort Study. Cancer Epidemiol Biomarkers Prev 2005; 14: 483–490.
Tsai YY, McGlynn KA, Hu Y, Cassidy AB, Arnold J, Engstrom PF et al. Genetic susceptibility and dietary patterns in lung cancer. Lung Cancer 2003; 41: 269–281.
Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet 2013; 113: 569–580.
Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012; 142: 1009–1018.
Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2005; 82: 163–173.
Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB . Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008; 168: 713–720.
Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 2001; 154: 1119–1125.
Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 2006; 355: 763–778.
Michaud DS, Midthune D, Hermansen S, Leitzmann M, Harlan LC, Kipnis V et al. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study. J Registry Manag 2005; 32: 70–78.
Thompson FE, Kipnis V, Midthune D, Freedman LS, Carroll RJ, Subar AF et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr 2008; 11: 183–195.
Mannisto S, Smith-Warner SA, Spiegelman D, Albanes D, Anderson K, van den Brandt PA et al. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol Biomarkers Prev 2004; 13: 40–48.
Lam TK, Rotunno M, Lubin JH, Wacholder S, Consonni D, Pesatori AC et al. Dietary quercetin, quercetin-gene interaction, metabolic gene expression in lung tissue and lung cancer risk. Carcinogenesis 2010; 31: 634–642.
Murakami A, Ashida H, Terao J . Multitargeted cancer prevention by quercetin. Cancer Lett 2008; 269: 315–325.
Bertram JS . Dietary carotenoids, connexins and cancer: what is the connection? Biochem Soc Trans 2004; 32: 985–989.
van den Brandt PA, Goldbohm RA, van 't Veer P, Bode P, Dorant E, Hermus RJ et al. A prospective cohort study on selenium status and the risk of lung cancer. Cancer Res 1993; 53: 4860–4865.
Knekt P, Marniemi J, Teppo L, Heliovaara M, Aromaa A . Is low selenium status a risk factor for lung cancer? Am J Epidemiol 1998; 148: 975–982.
Freudenheim JL, Ritz J, Smith-Warner SA, Albanes D, Bandera EV, van den Brandt PA et al. Alcohol consumption and risk of lung cancer: a pooled analysis of cohort studies. Am J Clin Nutr 2005; 82: 657–667.
Dallongeville J, Marecaux N, Fruchart JC, Amouyel P . Cigarette smoking is associated with unhealthy patterns of nutrient intake: a meta-analysis. J Nutr 1998; 128: 1450–1457.
Kipnis V, Subar AF, Midthune D, Freedman LS, Ballard-Barbash R, Troiano RP et al. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol 2003; 158: 14–21. Discussion 22-16.
Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol 2003; 158: 1–13.
Acknowledgements
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Anic, G., Park, Y., Subar, A. et al. Index-based dietary patterns and risk of lung cancer in the NIH–AARP diet and health study. Eur J Clin Nutr 70, 123–129 (2016). https://doi.org/10.1038/ejcn.2015.122
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2015.122
This article is cited by
-
Diet quality and lung cancer incidence in a low-income population in the United States
British Journal of Cancer (2023)
-
Dietary Patterns and Risk of Lung Cancer: A Systematic Review and Meta-Analyses of Observational Studies
Current Nutrition Reports (2023)
-
An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer
European Journal of Nutrition (2021)
-
Dietary quality using four dietary indices and lung cancer risk: the Golestan Cohort Study (GCS)
Cancer Causes & Control (2021)
-
Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies
Molecular Psychiatry (2019)