Abstract
Background/objectives:
A lower eating frequency (EF) has been suggested to be important in the development of cardiovascular risk factors such as obesity and hyperlipidemia. However, the association between EF and blood pressure (BP) remains unclear.
Subjects/methods:
The aim of this study was to explore the association of EF with BP and hypertension after adjusting for confounding variables, including body mass index (BMI) and waist circumference (WC). This cross-sectional study used data from the Third Korean National Health and Nutrition Examination Survey. A total of 4625 subjects aged ⩾19 years were included. To explore the association of EF with BP and hypertension, we performed multiple linear regression analyses and multiple logistic regression analyses for survey design, respectively.
Results:
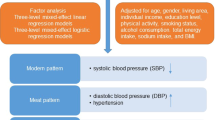
EF was inversely associated with systolic BP (SBP) and diastolic BP (DBP). As EF increased from ⩽2 to 3, 4 and ⩾5 times per day, estimated adjusted means of both SBP and DBP decreased, showing a significant linear trend independent of obesity (SBP: 120.66, 120.23, 119.18 and 117.92 mm Hg, respectively; P<0.001; DBP: 78.36, 77.78, 77.25 and 76.50 mm Hg, respectively; P=0.004). The inverse association between EF and hypertension was gradually attenuated and significant after adjustment for confounding variables including BMI and WC (P=0.040).
Conclusions:
This study suggests that lower EF is significantly associated with higher BP, which may be partially mediated by the effect of central obesity. Further prospective studies are needed to verify this causal relationship.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289: 2560–2572.
Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334: 13–18.
Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK . The progression from hypertension to congestive heart failure. JAMA 1996; 275: 1557–1562.
MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990; 335: 765–774.
Stamler J, Stamler R, Neaton JD . Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med 1993; 153: 598–615.
Lee JS, Park J, Kim J . Dietary factors related to hypertension risk in Korean adults—data from the Korean national health and nutrition examination survey III. Nutr Res Pract 2011; 5: 60–65.
Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons-Morton DG et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med 2001; 135: 1019–1028.
Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001; 344: 3–10.
Holmback I, Ericson U, Gullberg B, Wirfalt E . A high eating frequency is associated with an overall healthy lifestyle in middle-aged men and women and reduced likelihood of general and central obesity in men. Br J Nutr 2010; 104: 1065–1073.
Bachman JL, Phelan S, Wing RR, Raynor HA . Eating frequency is higher in weight loss maintainers and normal-weight individuals than in overweight individuals. J Am Diet Assoc 2011; 111: 1730–1734.
Jenkins DJ, Wolever TM, Vuksan V, Brighenti F, Cunnane SC, Rao AV et al. Nibbling versus gorging: metabolic advantages of increased meal frequency. N Engl J Med 1989; 321: 929–934.
Edelstein SL, Barrett-Connor EL, Wingard DL, Cohn BA . Increased meal frequency associated with decreased cholesterol concentrations; Rancho Bernardo, CA, 1984–1987. Am J Clin Nutr 1992; 55: 664–669.
Redondo MR, Ortega RM, Zamora MJ, Quintas ME, Lopez-Sobaler AM, Andres P et al. Influence of the number of meals taken per day on cardiovascular risk factors and the energy and nutrient intakes of a group of elderly people. Int J Vitam Nutr Res 1997; 67: 176–182.
Ritchie LD . Less frequent eating predicts greater BMI and waist circumference in female adolescents. Am J Clin Nutr 2012; 95: 290–296.
Smith KJ, Blizzard L, McNaughton SA, Gall SL, Dwyer T, Venn AJ . Daily eating frequency and cardiometabolic risk factors in young Australian adults: cross-sectional analyses. Br J Nutr 2011; 108: 1086–1094.
Benetou V, Bamia C, Trichopoulos D, Mountokalakis T, Psaltopoulou T, Trichopoulou A . The association of body mass index and waist circumference with blood pressure depends on age and gender: a study of 10,928 non-smoking adults in the Greek EPIC cohort. Eur J Epidemiol 2004; 19: 803–809.
Oda E, Kawai R . Body mass index is more strongly associated with hypertension than waist circumference in apparently healthy Japanese men and women. Acta Diabetol 2010; 47: 309–313.
Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr 2007; 85: 981–988.
Bertéus Forslund H, Klingström S, Hagberg H, Löndahl M, Torgerson JS, Lindroos AK . Should snacks be recommended in obesity treatment? A 1-year randomized clinical trial. Eur J Clin Nutr 2007; 62: 1308–1317.
Poston WSC, Haddock CK, Pinkston MM, Pace P, Karakoc ND, Reeves RS et al. Weight loss with meal replacement and meal replacement plus snacks: a randomized trial. Int J Obes 2005; 29: 1107–1114.
Korea Center for Disease Control and Prevention. The Third Korea National Health and Nutrition Examination Survey (KNHANES III): Analytic Guidelines 2006, Available at http://knhanes.cdc.go.kr/ (accessed 18 May 2011).
Park S-H, Lee K-S, Park H-Y . Dietary carbohydrate intake is associated with cardiovascular disease risk in Korean: analysis of the third Korea National Health and Nutrition Examination Survey (KNHANES III). Int J Cardiol 2010; 139: 234–240.
Karvetti RL, Knuts LR . Validity of the 24-hour dietary recall. J Am Diet Assoc 1985; 85: 1437–1442.
Biró G, Hulshof KF, Ovesen L, Amorim Cruz JA . Selection of methodology to assess food intake. Eur J Clin Nutr 2002; 56 (Suppl 2), S25–S32.
Madden JP, Goodman SJ, Guthrie HA . Validity of the 24-hr. recall. Analysis of data obtained from elderly subjects. J Am Diet Assoc 1976; 68: 143–147.
Kant AK . Indexes of overall diet quality: a review. J Am Diet Assoc 1996; 96: 785–791.
The World Health Organization Western Pacific Region, The International Association for the Study of Obesity, and The International Obesity Task Force. The Asia-Pacific Perspective: Redefining Obesity and its Treatment. Health Communications Australia Pty Limited: Sydney, Australia, 2000.
Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–1395.
Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract 2007; 75: 72–80.
Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH . Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med 1995; 155: 701–709.
Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA 2002; 288: 1882–1888.
Barba G, Troiano E, Russo P, Siani A . Total fat, fat distribution and blood pressure according to eating frequency in children living in southern Italy: the ARCA project. Int J Obes (Lond) 2006; 30: 1166–1169.
Ma Y, Bertone ER, Stanek 3rd EJ, Reed GW, Hebert JR, Cohen NL et al. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol 2003; 158: 85–92.
Kim S, Goh E, Lee DR, Park MS . The association between eating frequency and metabolic syndrome. Korean J Health Promot 2011; 11: 9–17.
Ruidavets JB, Bongard V, Bataille V, Gourdy P, Ferrieres J . Eating frequency and body fatness in middle-aged men. Int J Obes Relat Metab Disord 2002; 26: 1476–1483.
Frisoli TM, Schmieder RE, Grodzicki T, Messerli FH . Beyond salt: lifestyle modifications and blood pressure. Eur Heart J 2011; 32: 3081–3087.
Sheehan MT, Jensen MD . Metabolic complications of obesity. Pathophysiologic considerations. Med Clin N Am 2000; 84: 363–385. vi.
Jenkins DJ, Ocana A, Jenkins AL, Wolever TM, Vuksan V, Katzman L et al. Metabolic advantages of spreading the nutrient load: effects of increased meal frequency in non-insulin-dependent diabetes. Am J Clin Nutr 1992; 55: 461–467.
Sierra-Johnson J, Unden AL, Linestrand M, Rosell M, Sjogren P, Kolak M et al. Eating meals irregularly: a novel environmental risk factor for the metabolic syndrome. Obesity (Silver Spring) 2008; 16: 1302–1307.
Tack CJ, Smits P, Willemsen JJ, Lenders JW, Thien T, Lutterman JA . Effects of insulin on vascular tone and sympathetic nervous system in NIDDM. Diabetes 1996; 45: 15–22.
Zizza CA, Arsiwalla DD, Ellison KJ . Contribution of snacking to older adults' vitamin, carotenoid, and mineral intakes. J Am Diet Assoc 2010; 110: 768–772.
Shim E, Ryu HJ, Hwang J, Kim SY, Chung EJ . Dietary sodium intake in young Korean adults and its relationship with eating frequency and taste preference. Nutr Res Pract 2013; 7: 192–198.
The Korean Nutrition Society. Dietary Reference Intakes for Koreans, 1st revision. The Korean Nutrition Society: Seoul, Republic of Korea, 2010.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 2012-0000998). M-SP, H-JY and SK conceived the study, and G-HP and JHY helped design the study. G-HP, SK and SHC analyzed data and performed statistical analysis. M-SP and SK drafted the manuscript, and SHC, JHY and H-JY helped with the revision of the manuscript. M-SP, H-JY and SK have primary responsibility for the final content. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
Kim, S., Park, GH., Yang, J. et al. Eating frequency is inversely associated with blood pressure and hypertension in Korean adults: analysis of the Third Korean National Health and Nutrition Examination Survey. Eur J Clin Nutr 68, 481–489 (2014). https://doi.org/10.1038/ejcn.2014.9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2014.9
Keywords
This article is cited by
-
Eating patterns in Korean adults, 1998–2018: increased energy contribution of ultra-processed foods in main meals and snacks
European Journal of Nutrition (2024)
-
Eating patterns of Australian adults: associations with blood pressure and hypertension prevalence
European Journal of Nutrition (2019)
-
Association of meal frequency with metabolic syndrome in Korean adults: from the Korea National Health and Nutrition Examination Survey (KNHANES)
Diabetology & Metabolic Syndrome (2017)