Abstract
Background/Objectives:
Previous studies support the glucose-lowering effect of vinegar. However, the effect of vinegar on muscle glucose metabolism and endothelial function has not been studied in humans. This open, randomized, crossover, placebo-controlled study aims to investigate the effects of vinegar on muscle glucose metabolism, endothelial function and circulating lipid levels in subjects with impaired glucose tolerance (IGT) using the arteriovenous difference technique.
Subjects/Methods:
Eight subjects with IGT (4 males, age 46±10 years, body mass index 30±5) were randomised to consume 0.50 mmol vinegar (6% acetic acid) or placebo before a mixed meal. Plasma samples were taken for 300 min from the radial artery and the forearm vein for measurements of glucose, insulin, triglycerides, non-esterified fatty acids (NEFAs) and glycerol. Muscle blood flow was measured with strain gauge plethysmography. Glucose flux was calculated as the arteriovenous difference of glucose multiplied by the blood flow rates.
Results:
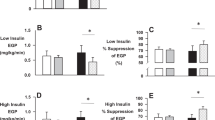
Vinegar compared with placebo: (1) decreased arterial plasma insulin (Poverall<0.001; P75 min=0.014, β=−42), (2) increased forearm blood flow (Poverall<0.001; P240 min=0.011, β=1.53; P300 min=0.023, β=1.37), (3) increased muscle glucose uptake (Poverall<0.001; P60 min=0.029, β=2.78) and (4) decreased arterial plasma triglycerides (Poverall=0.005; P240 min<0.001, β=−344; P300 min<0.001, β=−373), without changing NEFA and glycerol.
Conclusions:
In individuals with IGT, vinegar ingestion before a mixed meal results in an enhancement of muscle blood flow, an improvement of glucose uptake by the forearm muscle and a reduction of postprandial hyperinsulinaemia and hypertriglyceridaemia. From this point of view, vinegar may be considered beneficial for improving insulin resistance and metabolic abnormalities in the atherogenic prediabetic state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M . Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339: 229–234.
Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A . Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999; 22: 920–924.
Mykkanen L, Kuusisto J, Pyorala K, Laakso M . Cardiovascular disease risk factors as predictors of type 2 (non-insulin-dependent) diabetes mellitus in elderly subjects. Diabetologia 1993; 36: 553–559.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403.
Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343–1350.
Johnston CS, Gaas CA . Vinegar: medicinal uses and antiglycemic effect. MedGenMed 2006; 8: 61.
Johnston CS, Buller AJ . Vinegar and peanut products as complementary foods to reduce postprandial glycemia. J Am Diet Assoc 2005; 105: 1939–1942.
Johnston CS, Steplewska I, Long CA, Harris LN, Ryals RH . Examination of the antiglycemic properties of vinegar in healthy adults. Ann Nutr Metab 2010; 56: 74–79.
Leeman M, Ostman E, Bjorck I . Vinegar dressing and cold storage of potatoes lowers postprandial glycaemic and insulinaemic responses in healthy subjects. Eur J Clin Nutr 2005; 59: 1266–1271.
Ostman E, Granfeldt Y, Persson L, Bjorck I . Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr 2005; 59: 983–988.
Sugiyama M, Tang AC, Wakaki Y, Koyama W . Glycemic index of single and mixed meal foods among common Japanese foods with white rice as a reference food. Eur J Clin Nutr 2003; 57: 743–752.
Johnston CS, Kim CM, Buller AJ . Vinegar improves insulin sensitivity to a high-carbohydrate meal in subjects with insulin resistance or type 2 diabetes. Diabetes Care 2004; 27: 281–282.
Mitrou P, Raptis AE, Lambadiari V, Boutati E, Petsiou E, Spanoudi F et al. Vinegar decreases postprandial hyperglycemia in patients with type 1 diabetes. Diabetes Care 2010; 33: e27.
White AM, Johnston CS . Vinegar ingestion at bedtime moderates waking glucose concentrations in adults with well-controlled type 2 diabetes. Diabetes Care. 2007; 30: 2814–2815.
Johnston CS, White AM, Kent SM . Preliminary evidence that regular vinegar ingestion favorably influences hemoglobin A1c values in individuals with type 2 diabetes mellitus. Diabetes Res Clin Pract 2009; 84: e15–e17.
Liatis S, Grammatikou S, Poulia KA, Perrea D, Makrilakis K, Diakoumopoulou E et al. Vinegar reduces postprandial hyperglycaemia in patients with type II diabetes when added to a high, but not to a low, glycaemic index meal. Eur J Clin Nutr 2010; 64: 727–732.
Petsiou EI, Mitrou PI, Raptis SA, Dimitriadis GD . Effect and mechanisms of action of vinegar on glucose metabolism, lipid profile, and body weight. Nutr Rev 2014; 72: 651–661.
Cocchi M, Durante C, Grandi M, Lambertini P, Manzini D, Marchetti A . Simultaneous determination of sugars and organic acids in aged vinegars and chemometric data analysis. Talanta 2006; 69: 1166–1175.
Liljeberg H, Bjorck I . Delayed gastric emptying rate may explain improved glycaemia in healthy subjects to a starchy meal with added vinegar. Eur J Clin Nutr 1998; 52: 368–371.
Hlebowicz J, Darwiche G, Bjorgell O, Almer LO . Effect of apple cider vinegar on delayed gastric emptying in patients with type 1 diabetes mellitus: a pilot study. BMC Gastroenterol 2007; 7: 46.
Ogawa N, Satsu H, Watanabe H, Fukaya M, Tsukamoto Y, Miyamoto Y et al. Acetic acid suppresses the increase in disaccharidase activity that occurs during culture of caco-2 cells. J Nutr 2000; 130: 507–513.
Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA . Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract 2011; 93: S52–S59.
Dimitriadis G, Mitrou P, Lambadiari V, Boutati E, Maratou E, Panagiotakos DB et al. Insulin action in adipose tissue and muscle in hypothyroidism. J Clin Endocrinol Metab 2006; 91: 4930–4937.
Lambadiari V, Mitrou P, Maratou E, Raptis A, Raptis SA, Dimitriadis G . Increases in muscle blood flow after a mixed meal are impaired at all stages of type 2 diabetes. Clin Endocrinol (Oxf) 2012; 76: 825–830.
Mitrou P, Boutati E, Lambadiari V, Maratou E, Papakonstantinou A, Komesidou V et al. Rates of glucose uptake in adipose tissue and muscle in vivo after a mixed meal in women with morbid obesity. J Clin Endocrinol Metab 2009; 94: 2958–2961.
Dimitriadis G, Mitrou P, Lambadiari V, Boutati E, Maratou E, Koukkou E et al. Glucose and lipid fluxes in the adipose tissue after meal ingestion in hyperthyroidism. J Clin Endocrinol Metab 2006; 91: 1112–1118.
Fushimi T, Sato Y . Effect of acetic acid feeding on the circadian changes in glycogen and metabolites of glucose and lipid in liver and skeletal muscle of rats. Br J Nutr 2005; 94: 714–719.
Fushimi T, Tayama K, Fukaya M, Kitakoshi K, Nakai N, Tsukamoto Y et al. The efficacy of acetic acid for glycogen repletion in rat skeletal muscle after exercise. Int J Sports Med 2002; 23: 218–222.
Sakakibara S, Yamauchi T, Oshima Y, Tsukamoto Y, Kadowaki T . Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A(y) mice. Biochem Biophys Res Commun 2006; 344: 597–604.
Fushimi T, Suruga K, Oshima Y, Fukiharu M, Tsukamoto Y, Goda T . Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. Br J Nutr 2006; 95: 916–924.
Lozano J, Juárez-Flores B, Pinos-Rodríguez J, Aguirre-Rivera J, Álvarez-Fuentes G . Supplementary effects of vinegar on body weight and blood metabolites in healthy rats fed conventional diets and obese rats fed high-caloric diets. J Med Plants Res 2012; 6: 4135–4141.
Moon Y, Choi D, Oh S, Song Y, Cha Y . Effects of persimmon-vinegar on lipid and carnitine profiles in mice. Food Sci Biotechnol 2010; 19: 343–348.
Setorki M, Asgary S, Akram E, Rohani AH, Khazzaei M . Acute effects of vinegar intake on some biochemical risk factors of atherosclerosis in hypercholesterolemic rabbits. Lipids Health Dis 2010; 9: 10.
Yamashita H, Fujisawa K, Ito E, Idei S, Kawaguchi N, Kimoto M et al. Improvement of obesity and glucose tolerance by acetate in type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci Biotechnol Biochem 2007; 71: 1236–1243.
Beheshti Z, Chan Y, Nia H, Hajihosseini F, Nazari R, Shaabani M et al. Influence of apple cider vinegar on blood lipids. Life Sci J 2012; 9: 2431–2440.
Kondo T, Kishi M, Fushimi T, Ugajin S, Kaga T . Vinegar intake reduces body weight, body fat mass, and serum triglyceride levels in obese Japanese subjects. Biosci Biotechnol Biochem 2009; 73: 1837–1843.
Yamashita H, Maruta H, Jozuka M, Kimura R, Iwabuchi H, Yamato M et al. Effects of acetate on lipid metabolism in muscles and adipose tissues of type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci Biotechnol Biochem 2009; 73: 570–576.
Coppack SW, Frayn KN, Humphreys SM, Whyte PL, Hockaday TD . Arteriovenous differences across human adipose and forearm tissues after overnight fast. Metabolism 1990; 39: 384–390.
American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36: S11–S66.
Coppack SW, Fisher RM, Gibbons GF, Humphreys SM, McDonough MJ, Potts JL et al. Postprandial substrate deposition in human forearm and adipose tissues in vivo. Clin Sci (Lond) 1990; 79: 339–348.
Frayn KN, Shadid S, Hamlani R, Humphreys SM, Clark ML, Fielding BA et al. Regulation of fatty acid movement in human adipose tissue in the postabsorptive-to-postprandial transition. Am J Physiol 1994; 266: E308–E317.
Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981; 34: 362–366.
Sakakibara S, Murakami R, Takahashi M, Fushimi T, Murohara T, Kishi M et al. Vinegar intake enhances flow-mediated vasodilatation via upregulation of endothelial nitric oxide synthase activity. Biosci Biotechnol Biochem 2010; 74: 1055–1061.
Waller AP, Geor RJ, Spriet LL, Heigenhauser GJ, Lindinger MI . Oral acetate supplementation after prolonged moderate intensity exercise enhances early muscle glycogen resynthesis in horses. Exp Physiol 2009; 94: 888–898.
Budak NH, Kumbul Doguc D, Savas CM, Seydim AC, Kok Tas T, Ciris MI et al. Effects of apple cider vinegars produced with different techniques on blood lipids in high-cholesterol-fed rats. J Agric Food Chem 2011; 59: 6638–6644.
Kondo T, Kishi M, Fushimi T, Kaga T . Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J Agric Food Chem 2009; 57: 5982–5986.
Moon YJ, Cha YS . Effects of persimmon-vinegar on lipid metabolism and alcohol clearance in chronic alcohol-fed rats. J Med Food 2008; 11: 38–45.
Shishehbor F, Mansoori A, Sarkaki AR, Jalali MT, Latifi SM . Apple cider vinegar attenuates lipid profile in normal and diabetic rats. Pak J Biol Sci 2008; 11: 2634–2638.
Kahraman N, Mesci B, Oguz A, Tamer G, Kahraman C, Sagun G et al. The effect of vinegar on postprandial glycemia. Does the amount matter? Acta Endo (Buc) 2011; 7: 577–558.
Panetta C, Jonk Y, Shapiro A . Prospective randomized clinical trial evaluating the impact of vinegar on lipids in non-diabetics. World J Cardiovasc Dis 2013; 3: 191–196.
Haffner SM, Mykkanen L, Festa A, Burke JP, Stern MP . Insulin-resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects: implications for preventing coronary heart disease during the prediabetic state. Circulation 2000; 101: 975–980.
Acknowledgements
We are grateful to E Pappas and I Kosmopoulou for technical support, and V Frangaki and RN for help with experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mitrou, P., Petsiou, E., Papakonstantinou, E. et al. The role of acetic acid on glucose uptake and blood flow rates in the skeletal muscle in humans with impaired glucose tolerance. Eur J Clin Nutr 69, 734–739 (2015). https://doi.org/10.1038/ejcn.2014.289
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2014.289
This article is cited by
-
Metagenetic analysis of the bacterial diversity of Kazakh koumiss and assessment of its anti-Candida albicans activity
World Journal of Microbiology and Biotechnology (2024)
-
Lipid and metabolite correlation networks specific to clinical and biochemical covariate show differences associated with sexual dimorphism in a cohort of nonagenarians
GeroScience (2022)
-
Short-term Cudrania tricuspidata fruit vinegar administration attenuates obesity in high-fat diet-fed mice by improving fat accumulation and metabolic parameters
Scientific Reports (2020)
-
Optimization of a Process for Preparation of Base Wine for Cider Vinegar Production
Proceedings of the National Academy of Sciences, India Section B: Biological Sciences (2019)
-
Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice
The ISME Journal (2016)