Abstract
Background/Objectives:
Chronic kidney disease (CKD) is a major health concern associated with increased risk of cardiovascular disease, morbidity and mortality. Current CKD practice guidelines overlook dietary fiber, which is chronically low in the renal diet. However, increasing dietary fiber has been proposed to ameliorate the progress of CKD. We therefore conducted a systematic review and meta-analysis on the effect of dietary fiber intake on serum urea and creatinine as classical markers of renal health in individuals with CKD.
Subjects/Methods:
We searched MEDLINE, EMBASE, CINHAL and the Cochrane Library for relevant clinical trials with a follow-up ⩾7 days. Data were pooled by the generic inverse variance method using random-effects models and expressed as mean difference (MD) with 95% confidence intervals (95% CIs). Heterogeneity was assessed by the Cochran Q statistic and quantified by I2.
Results:
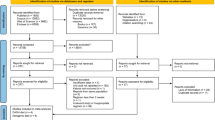
A total of 14 trials involving 143 participants met the eligibility criteria. Dietary fiber supplementation significantly reduced serum urea and creatinine levels in the primary pooled analyses (MD, −1.76 mmol/l (95% CI, −3.00, −0.51), P<0.01 and MD, −22.83 mmol/l (95% CI, −42.63, −3.02), P=0.02, respectively) with significant evidence of interstudy heterogeneity only in the analysis of serum urea.
Conclusions:
This is the first study to summarize the potential beneficial effects of dietary fiber in the CKD population demonstrating a reduction in serum urea and creatinine, as well as highlighting the lack of clinical trials on harder end points. Larger, longer, higher-quality clinical trials measuring a greater variety of uremic toxins in CKD are required (NCT01844882).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Kidney Diseases. Am J Kidney Dis 2007; 2: S1–S180
Fouque D, Vennegoor M, ter Wee P, Wanner C, Basci A, Canaud B et al. EBPG guideline on nutrition. Nephrol Dial Transplant. 2007; 22: ii45–ii87.
Harris D, Thomas M, Johnson D, Nicholls K, Gillin A . Caring for Australasians with renal I. The CARI guidelines. Prevention of progression of kidney disease. Nephrology 2006; 11: S2–197.
Levin A, Hemmelgarn B, Culleton B, Tobe S, McFarlane P, Ruzicka M et al. Guidelines for the management of chronic kidney disease. CMAJ 2008; 179: 1154–1162.
Kopple JD . National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis 2001; 37: S66–S70.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO Clinical Practice Guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013; 3: 1.
Stephen AM, Cummings JH . Mechanism of action of dietary fibre in the human colon. Nature 1980; 284: 283–284.
Meijers BK, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P . p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant 2010; 25: 219–224.
Meyer TW, Hostetter TH . Uremia. N Engl J Med 2007; 357: 1316–1325.
Schepers E, Glorieux G, Vanholder R . The gut: the forgotten organ in uremia? Blood Purif 2010; 29: 130–136.
Evenepoel P, Meijers BK, Bammens BR, Verbeke K . Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl 2009; 114: S12–S19.
Sánchez-Muniz F . Dietary fiber and cardiovascular health. Nutricion Hospitalaria 2012; 27: 31–45.
Gopinath B, Harris DC, Flood VM, Burlutsky G, Brand-Miller J, Mitchell P . Carbohydrate nutrition is associated with the 5-year incidence of chronic kidney disease. J Nutr 2011; 141: 433–439.
Diaz-Lopez A, Bullo M, Basora J, Martinez-Gonzalez MA, Guasch-Ferre M, Estruch R et al. Cross-sectional associations between macronutrient intake and chronic kidney disease in a population at high cardiovascular risk. Clin Nutr 2013; 32: 606–612.
Krishnamurthy VM, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL et al. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 2012; 81: 300–306.
Higgins JP GS, ed. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. The Cochrane Collaboration; 2011 Accessed at www.cochrane-handbookorg on 22 December 2011 [Internet].
Moher D CD, Eastwood S, Olkin I, Rennie D, Stroup DF . Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999; 354: 1896–1900.
Moher D LA, Tetzlaff J, Altman DG . PRISMA Group Preferred statement. Ann Intern Med 2009; 151: 264–269
Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N . Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 2006; 59: 7–10
Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A . Meta-analyses involving cross-over trials: methodological issues. nt J Epidemiol. 2002; 31: 140–149.
Begg CB . A measure to aid in the interpretation of published clinical trials. Stat Med 1985; 4: 1–9.
Egger M, Davey Smith G, Schneider M, Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634.
Salmean YA, Segal MS, Langkamp-Henken B, Canales MT, Zello GA, Dahl WJ . Foods with added fiber lower serum creatinine levels in patients with chronic kidney disease. J Ren Nutr 2013; 23: e29–e32.
Younes H, Egret N, Hadj-Abdelkader M, Remesy C, Demigne C, Gueret C et al. Fermentable carbohydrate supplementation alters nitrogen excretion in chronic renal failure. J Ren Nutr 2006; 16: 67–74.
Rampton DS, Cohen SL, Crammond VD, Gibbons J, Lilburn MF, Rabet JY et al. Treatment of chronic renal failure with dietary fiber. Clin Nephrol 1984; 21: 159–163.
Bliss DZ, Stein TP, Schleifer CR, Settle RG . Supplementation with gum arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am J Clin Nutr 1996; 63: 392–398.
Rivellese A, Parillo M, Giacco A, De Marco F, Riccardi G . A fiber-rich diet for the treatment of diabetic patients with chronic renal failure. Diabetes Care 1985; 8: 620–621.
Parillo M, Riccardi G, Pacioni D, Iovine C, Contaldo F, Isernia C et al. Metabolic consequences of feeding a high-carbohydrate, high-fiber diet to diabetic patients with chronic kidney failure. Am J Clin Nutr 1988; 48: 255–259.
Burgess MB, Littlefield D . Effect of wheat bran supplementation on colonic function and serum mineral levels in chronic renal failure hemodialysis patients. Dial Transpl 1987; 16: 184–189.
Yatzidis H, Koutsicos D, Digenis P . Oral locust bean gum therapy of uremia - favorable effects on biological abnormalities and hypertension. Dial Transpl 1980; 9: 313–317.
Miura M, Nomoto Y, Sakai H . Short term effect of lactulose therapy in patients with chronic renal failure. Tokai J Exp Clin Med 1989; 14: 29–34.
Miyazaki M, Aoyagi K, Tojo S . [Lactulose therapy for chronic renal failure]. Nihon Jinzo Gakkai shi 1984; 26: 1091–1098.
Pender FT . The effect of increasing the dietary fibre content of diets of patients with chronic renal failure treated by haemodialysis at home. J Hum Nutr Diet 1989; 2: 423–427.
Vince AJ, McNeil NI, Wager JD, Wrong OM . The effect of lactulose, pectin, arabinogalactan and cellulose on the production of organic acids and metabolism of ammonia by intestinal bacteria in a faecal incubation system. Br J Nutr 1990; 63: 17–26.
Levrat MA, Behr SR, Remesy C, Demigne C . Effects of soybean fiber on cecal digestion in rats previously adapted to a fiber-free diet. J Nutr 1991; 121: 672–678.
Younes H, Garleb K, Behr S, Remesy C, Demigne C . Fermentable fibers or oligosaccharides reduce urinary nitrogen excretion by increasing urea disposal in the rat cecum. J Nutr 1995; 125: 1010–1016.
Younes H DC, Behr S, Rémésy C . Resistant starch exerts an uremia lowering effect by enhancing urea disposal in the large intestine. Nutr Res 1995; 15: 1199–1210.
Young GP, Gibson PR . Butyrate and the human cancer cell. In: Cummings JH, Rombeau JL, Sakata T (eds). Physiological and Clinical Aspects of Short-chain fatty acids. Cambridge University Press: Cambridge, UK, 1995,, pp 319–336.
Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A . Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 2011; 17: 1519–1528.
Bauer-Marinovic M, Florian S, Muller-Schmehl K, Glatt H, Jacobasch G . Dietary resistant starch type 3 prevents tumor induction by 1,2-dimethylhydrazine and alters proliferation, apoptosis and dedifferentiation in rat colon. Carcinogenesis 2006; 27: 1849–1859.
Demigne C, Remesy C . Influence of unrefined potato starch on cecal fermentations and volatile fatty acid absorption in rats. J Nutr 1982; 112: 2227–2234.
Aufreiter S, Kim JH, O'Connor DL . Dietary oligosaccharides increase colonic weight and the amount but not concentration of bacterially synthesized folate in the colon of piglets. J Nutr 2011; 141: 366–372.
Jahns F, Wilhelm A, Jablonowski N, Mothes H, Radeva M, Wolfert A et al. Butyrate suppresses mRNA increase of osteopontin and cyclooxygenase-2 in human colon tumor tissue. Carcinogenesis 2011; 32: 913–920.
Demigne C, Levrat MA, Remesy C . Effects of feeding fermentable carbohydrates on the cecal concentrations of minerals and their fluxes between the cecum and blood plasma in the rat. J Nutr 1989; 119: 1625–1630.
Levrat MA, Rémésy C, Demigné C . Very acidic fermentations in the rat cecum during adaptation to a diet rich in amylase-resistant starch (crude potato starch). Nutr Biochem 1991; 2: 31–36.
Moran BJ, Jackson AA . 15N-urea metabolism in the functioning human colon: luminal hydrolysis and mucosal permeability. Gut 1990; 31: 454–457.
Turner JR, Cohen DE, Mrsny RJ, Madara JL . Noninvasive in vivo analysis of human small intestinal paracellular absorption: regulation by Na+-glucose cotransport. Dig Dis Sci 2000; 45: 2122–2126.
Jones JD, Burnett PC . Creatinine metabolism in humans with decreased renal function: creatinine deficit. Clin Chem 1974; 20: 1204–1212.
Dunn SR, Gabuzda GM, Superdock KR, Kolecki RS, Schaedler RW, Simenhoff ML . Induction of creatininase activity in chronic renal failure: timing of creatinine degradation and effect of antibiotics. Am J Kidney Dis 1997; 29: 72–77.
Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004; 15: 1307–1315.
Vuksan V, Jenkins DJ, Spadafora P, Sievenpiper JL, Owen R, Vidgen E et al. Konjac-mannan (glucomannan) improves glycemia and other associated risk factors for coronary heart disease in type 2 diabetes. A randomized controlled metabolic trial. Diabetes Care 1999; 22: 913–919.
Streppel MT, Arends LR, van 't Veer P, Grobbee DE, Geleijnse JM . Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med 2005; 165: 150–156.
Brown L, Rosner B, Willett WW, Sacks FM . Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 1999; 69: 30–42.
Schrijvers BF, De Vriese AS, Flyvbjerg A . From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev 2004; 25: 971–1010.
Blood Pressure Lowering Treatment Trialists Collaboration. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: meta-analysis of randomised controlled trials. BMJ 2013; 347: f5680.
Threapleton DE, Greenwood DC, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C et al. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 2013; 347: f6879.
He M, van Dam RM, Rimm E, Hu FB, Whole-grain Qi L . cereal fiber, bran, and germ intake and the risks of all-cause and cardiovascular disease-specific mortality among women with type 2 diabetes mellitus. Circulation 2010; 121: 2162–2168.
Liu S, Buring JE, Sesso HD, Rimm EB, Willett WC, Manson JE . A prospective study of dietary fiber intake and risk of cardiovascular disease among women. J Am Coll Cardiol 2002; 39: 49–56.
Jenkins DJ, Kendall CW, Vuksan V, Vidgen E, Parker T, Faulkner D et al. Soluble fiber intake at a dose approved by the US Food and Drug Administration for a claim of health benefits: serum lipid risk factors for cardiovascular disease assessed in a randomized controlled crossover trial. Am J Clin Nutr 2002; 75: 834–839.
Tosh SM . Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur J Clin Nutr 2013; 67: 310–317.
North CJ, Venter CS, Jerling JC . The effects of dietary fibre on C-reactive protein, an inflammation marker predicting cardiovascular disease. Eur J Clin Nutr 2009; 63: 921–933.
Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y . Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 2006; 69: 1081–1087.
Meijers BK, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P . Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 2008; 73: 1174–1180.
Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 2010; 25: 1183–1191.
Niwa T, Nomura T, Sugiyama S, Miyazaki T, Tsukushi S, Tsutsui S . The protein metabolite hypothesis, a model for the progression of renal failure: an oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int Suppl 1997; 62: S23–S28.
Meyer TW, Hostetter TH . Uremic solutes from colon microbes. Kidney Int 2012; 81: 949–954.
Rossi M, Klein K, Johnson DW, Campbell KL . Pre-, pro-, and synbiotics: do they have a role in reducing uremic toxins? A systematic review and meta-analysis. Int J Nephrol 2012; 2012: 673631.
Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 2011; 305: 1119–1127.
Putcha N, Allon M . Management of hyperkalemia in dialysis patients. Semin Dial 2007; 20: 431–439.
Acknowledgements
LC and PBD designed the research. LC and AM conducted the research. LC and AM analyzed the data. LC and PBD wrote the paper. LC, AM, JLS, DJAJ and PBD were responsible for critical revision of the manuscript. PBD had primary responsibility for final content. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
LC works as a casual Clinical Research Coordinator at GI Laboratories, Toronto, Canada. LC and AM have received research support from the Canadian Institutes of Health Research (CIHR). JLS has received research support from the CIHR, Calorie Control Council, The Coca-Cola Company (investigator initiated, unrestricted grant), Pulse Canada and The International Tree Nut Council Nutrition Research & Education Foundation. He has received travel funding, speaker fees and/or honoraria from the American Heart Association (AHA), American Society for Nutrition (ASN), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH), Canadian Diabetes Association (CDA), Canadian Nutrition Society (CNS), Calorie Control Council, Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD), International Life Sciences Institute (ILSI) North America, International Life Sciences Institute (ILSI) Brazil, Abbott Laboratories, Pulse Canada, Dr. Pepper Snapple Group and The Coca-Cola Company. He is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of both the Canadian Diabetes Association (CDA) and European Association for the study of Diabetes (EASD), as well as being on the American Society for Nutrition (ASN) writing panel for a scientific statement on the metabolic and nutritional effects of fructose, sucrose and high-fructose corn syrup. He is an unpaid scientific advisor for the International Life Science Institute (ILSI) North America, Food, Nutrition and Safety Program (FNSP). His wife is an employee of Unilever Canada. DJAJ holds an unrestricted grant from the Coca-Cola Company and has served on the scientific advisory board for or received research support, consultant fees or honoraria from Barilla, Solae, Unilever, Hain Celestial, Loblaws Supermarkets, Sanitarium Company, Herbalife International, Pacific Health Laboratories Inc, Metagenics/MetaProteomics, Bayer Consumer Care, Oldways Preservation Trust, The International Tree Nut Council Nutrition Research & Education, The Peanut Institute, Procter and Gamble Technical Centre Limited, Griffin Hospital for the development of the NuVal System, Pepsi Company, Soy Advisory Board of Dean Foods, Alpro Soy Foundation, Nutritional Fundamentals for Health, Pacific Health Laboratories, Kellogg’s, Quaker Oats, The Coca-Cola Sugar Advisory Board, Agrifoods and Agriculture Canada (AAFC), Canadian Agriculture Policy Institute (CAPI), Abbott Laboratories, the Almond Board of California, the California Strawberry Commission, Orafti, the Canola and Flax Councils of Canada, Pulse Canada and the Saskatchewan Pulse Growers. DJAJ also holds additional grant support from the Canadian Institutes of Health Research, Canadian Foundation for Innovation, Ontario Research Fund and Advanced Foods and Material Network. DJAJ’s spouse is a vice president and director of research at GI Laboratories (Toronto, Ontario, Canada). PBD declares no conflict of interest.
Additional information
This study was presented in part at the Canadian Nutrition Society conference, St. John’s Newfoundland, 5–7 June 2014.
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
Chiavaroli, L., Mirrahimi, A., Sievenpiper, J. et al. Dietary fiber effects in chronic kidney disease: a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr 69, 761–768 (2015). https://doi.org/10.1038/ejcn.2014.237
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2014.237
This article is cited by
-
The benefits of dietary fiber: the gastrointestinal tract and beyond
Pediatric Nephrology (2023)
-
Metagenome-wide analysis uncovers gut microbial signatures and implicates taxon-specific functions in end-stage renal disease
Genome Biology (2023)
-
Low adherence to CKD-specific dietary recommendations associates with impaired kidney function, dyslipidemia, and inflammation
European Journal of Clinical Nutrition (2021)
-
Healthy adult vegetarians have better renal function than matched omnivores: a cross-sectional study in China
BMC Nephrology (2020)
-
The effects of 16-weeks of prebiotic supplementation and aerobic exercise training on inflammatory markers, oxidative stress, uremic toxins, and the microbiota in pre-dialysis kidney patients: a randomized controlled trial-protocol paper
BMC Nephrology (2020)