Abstract
Background/Objectives:
Whey proteins have insulinogenic properties and the effect appears to be mediated from a postprandial plasma amino-acid (AA) response. The aim was to study the possible dose–response relationship between whey intake and glycaemic-, insulinaemic- and plasma AA responses.
Subjects/Methods:
Twelve healthy volunteers participated in the study. They were provided three whey protein drinks, containing 4.5, 9 or 18 g protein as breakfast meals in random order. All meals contained 25 g available carbohydrates (glucose). The same amount of glucose in water was used as reference.
Results:
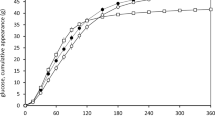
Linear dose–response relations were found between whey protein intake and postprandial glycaemia, insulinaemia and plasma AAs. The two highest doses, 18 g and 9 g, significantly reduced postprandial glycaemia (incremental area under the curve (iAUC) 0–120 min; P⩽0.05). The 18 g dose significantly increased the insulin response (iAUC 0–120 min; P⩽0.05). All measured plasma AAs (15 in total), except glutamic acid, responded in a dose-dependent way, and the 9 and 18 g doses resulted in significantly higher plasma levels of AAs compared with the reference.
Conclusions:
Whey protein affects glycaemia, insulinaemia and plasma AAs to a glucose load in a dose-dependent manner. Comparatively low doses of whey protein (9 g) reduced postprandial glycaemia significantly when added to a carbohydrate-rich meal.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Elwood PC, Pickering JE, Fehily AM . Milk and dairy consumption, diabetes and the metabolic syndrome: the Caerphilly prospective study. J Epidemiol Community Health 2007; 61: 695–698.
Malik VS, Sun Q, van Dam RM, Rimm EB, Willett WC, Rosner B et al. Adolescent dairy product consumption and risk of type 2 diabetes in middle-aged women. Am J Clin Nutr 2011; 94: 854–861.
Jakubowicz D, Froy O . Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and type 2 diabetes. J Nutr Biochem 2012; 24: 1–5.
Pfeuffer M, Schrezenmeir J . Milk and the metabolic syndrome. Obes Rev 2007; 8: 109–118.
Luhovyy BL, Akhavan T, Anderson GH . Whey proteins in the regulation of food intake and satiety. J Am Coll Nutr 2007; 26: 704S–712S.
Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IM . Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr 2004; 80: 1246–1253.
Nilsson M, Holst JJ, Bjorck IME . Metabolic effects of amino acid mixtures and whey protein in healthy subjects: studies using glucose-equivalent drinks. Am J Clin Nutr 2007; 85: 996–1004.
Salehi A, Gunnerud U, Muhammed S, Ostman E, Holst J, Bjorck I et al. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on beta-cells. Nutr Metab 2012; 9: 48.
Lan-Pidhainy X, Wolever TM . The hypoglycemic effect of fat and protein is not attenuated by insulin resistance. Am J Clin Nutr 2010; 91: 98–105.
Frid AH, Nilsson M, Holst JJ, Bjorck IM . Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr 2005; 82: 69–75.
Petersen BL, Ward LS, Bastian ED, Jenkins AL, Campbell J, Vuksan V . A whey protein supplement decreases post-prandial glycemia. Nutr J 2009; 8: 47.
Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G et al. Glycaemic index methodology. Nutr Res Rev 2005; 18: 145–171.
Gunnerud UJ, Heinzle C, Holst JJ, Ostman EM, Bjorck IM . Effects of pre-meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS One 2012; 7: e44731.
Gannon MC, Nuttall FQ . Amino acid ingestion and glucose metabolism-a review. IUBMB Life 2010; 62: 660–668.
Fajans SS, Floyd JC Jr., Knopf RF, Conn FW . Effect of amino acids and proteins on insulin secretion in man. Recent Prog Horm Res 1967; 23: 617–662.
Floyd JC Jr., Fajans SS, Conn JW, Knopf RF, Rull J . Stimulation of insulin secretion by amino acids. J Clin Invest 1966; 45: 1487–1502.
Newsholme P, Bender K, Kiely A, Brennan L . Amino acid metabolism, insulin secretion and diabetes. Biochem Soc Trans 2007; 35 (Pt 5), 1180–1186.
Flatt PR, Kwasowski P, Howland RJ, Bailey CJ . Gastric inhibitory polypeptide and insulin responses to orally administered amino acids in genetically obese hyperglycemic (ob/ob) mice. J Nutr 1991; 121: 1123–1128.
Thomas FB, Sinar D, Mazzaferri EL, Cataland S, Mekhjian HS, Caldwell JH et al. Selective release of gastric inhibitory polypeptide by intraduodenal amino acid perfusion in man. Gastroenterology 1978; 74: 1261–1265.
Poppitt SD, Proctor J, McGill AT, Wiessing KR, Falk S, Xin L et al. Low-dose whey protein-enriched water beverages alter satiety in a study of overweight women. Appetite 2011; 56: 456–464.
Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen MP et al. Dose-dependent satiating effect of whey relative to casein or soy. Physiol Behav 2009; 96: 675–682.
Veldhorst MAB, Nieuwenhuizen AG, Hochstenbach-Waelen A, Westerterp KR, Engelen MPKJ, Brummer RJM et al. A breakfast with alpha-lactalbumin, gelatin, or gelatin plus TRP lowers energy intake at lunch compared with a breakfast with casein, soy, whey, or whey-GMP. Clin Nutr 2009; 28: 147–155.
Acknowledgements
This study was funded by the Lund University Antidiabetic Food Center, a VINNOVA VINN Excellence Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Contributors: UJG coordinated the study and was involved in the study design, the collection and analysis of the data, statistical analysis and the evaluation and the writing of the paper. EMÖ was involved in the study design, interpretation of data and in writing the paper. IMEB was involved in the study design and for securing the funding and was involved in the evaluation and in writing the paper. All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Gunnerud, U., Östman, E. & Björck, I. Effects of whey proteins on glycaemia and insulinaemia to an oral glucose load in healthy adults; a dose–response study. Eur J Clin Nutr 67, 749–753 (2013). https://doi.org/10.1038/ejcn.2013.88
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2013.88
Keywords
This article is cited by
-
Effects of supplementation with milk protein on glycemic parameters: a GRADE-assessed systematic review and dose–response meta-analysis
Nutrition Journal (2023)
-
Whey protein improves glycemia during an oral glucose tolerance test compared to vigorous-intensity aerobic exercise in young adult men
BMC Sports Science, Medicine and Rehabilitation (2022)
-
Comparison of ingesting a food bar containing whey protein and isomalto-oligosaccharides to carbohydrate on performance and recovery from an acute bout of resistance-exercise and sprint conditioning: an open label, randomized, counterbalanced, crossover pilot study
Journal of the International Society of Sports Nutrition (2019)
-
Glycaemic and insulinaemic impact of oats soaked overnight in milk vs. cream of rice with and without sugar, nuts, and seeds: a randomized, controlled trial
European Journal of Clinical Nutrition (2019)
-
Effects of a casein hydrolysate versus intact casein on gastric emptying and amino acid responses
European Journal of Nutrition (2019)