Abstract
Background/Objective:
Nut consumption has been found to decrease risk of coronary heart disease and diabetes and to promote healthy body weights possibly related to their favorable macronutrient profile. We therefore assessed the effect of pistachios on postprandial glucose and insulin levels, gut hormones related to satiety and endothelial function.
Subjects/Methods:
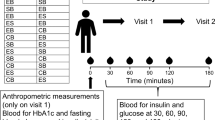
In this randomized crossover study, 20 subjects with metabolic syndrome consumed five study meals over 5–10 weeks. The meals differed in fat type and quantity, but were matched according to available carbohydrates (CHOs). Three meals had 50 g available CHO: white bread (WB50g), white bread, butter and cheese (WB+B+Ch) and white bread and pistachios (WB+P). Two meals had 12 g available CHO: white bread (WB12g) and pistachios (P).
Results:
Within each group of available CHO meals, postprandial glucose levels were the highest following the white bread-only meals, and glucose response was significantly attenuated when butter and cheese or pistachios were consumed (P<0.05). Postprandial insulin levels were highest after the WB+B+Ch meal (P<0.05), but did not differ between the white bread-only and pistachio meals. Both endothelial function (reactive hyperemia index) and arterial stiffness (augmentation index) significantly increased after the white bread-only meals compared with the WB+B+Ch meal (all P<0.05). Insulin secretagogue levels were higher when butter and cheese or pistachios were consumed than when white bread only was consumed (P<0.05).
Conclusions:
Compared with white bread, pistachio consumption reduced postprandial glycemia, increased glucagon-like-peptide levels and may have insulin-sparing properties. These effects could be beneficial for individuals with diabetes and metabolic syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hu FB, Stampfer MJ, Manson JE, Rimm EB, Colditz GA, Rosner BA et al. Frequent nut consumption and risk of coronary heart disease in women: prospective cohort study. BMJ 1998; 317: 1341–1345.
Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB . Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 2002; 288: 2554–2560.
Li TY, Brennan AM, Wedick NM, Mantzoros C, Rifai N, Hu FB . Regular consumption of nuts is associated with a lower risk of cardiovascular disease in women with type 2 diabetes. J Nutr 2009; 139: 1333–1338.
Mente A, de Koning L, Shannon HS, Anand SS . A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med 2009; 169: 659–669.
Jenkins DJ, Kendall CW, Marchie A, Parker TL, Connelly PW, Qian W et al. Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation 2002; 106: 1327–1332.
Tapsell LC, Gillen LJ, Patch CS, Batterham M, Owen A, Bare M et al. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabet Care 2004; 27: 2777–2783.
Griel AE, Kris-Etherton PM . Tree nuts and the lipid profile: a review of clinical studies. Br J Nutr 2006; 96 (Suppl 2), S68–S78.
Kocyigit A, Koylu AA, Keles H . Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutr Metab Cardiovasc Dis 2006; 16: 202–209.
Sheridan MJ, Cooper JN, Erario M, Cheifetz CE . Pistachio nut consumption and serum lipid levels. J Am Coll Nutr 2007; 26: 141–148.
Gebauer SK, West SG, Kay CD, Alaupovic P, Bagshaw D, Kris-Etherton PM . Effects of pistachios on cardiovascular disease risk factors and potential mechanisms of action: a dose–response study. Am J Clin Nutr 2008; 88: 651–659.
Tapsell LC, Batterham MJ, Teuss G, Tan SY, Dalton S, Quick CJ et al. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr 2009; 63: 1008–1015.
Kay CD, Gebauer SK, West SG, Kris-Etherton PM . Pistachios increase serum antioxidants and lower serum oxidized-LDL in hypercholesterolemic adults. J Nutr 2010; 140: 1093–1098.
Jiang R, Jacobs Jr. DR, Mayer-Davis E, Szklo M, Herrington D, Jenny NS et al. Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am J Epidemiol 2006; 163: 222–231.
Ros E . Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nutr 2009; 89 (Suppl 5), 1649s–1656s.
Sari I, Baltaci Y, Bagci C, Davutoglu V, Erel O, Celik H et al. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: a prospective study. Nutrition 2010; 26: 399–404.
Cortes B, Nunez I, Cofan M, Gilabert R, Perez-Heras A, Casals E et al. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J Am Coll Cardiol 2006; 48: 1666–1671.
Jenkins DJ, Kendall CW, Josse AR, Salvatore S, Brighenti F, Augustin LS et al. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr 2006; 136: 2987–2992.
Josse AR, Kendall CW, Augustin LS, Ellis PR, Jenkins DJ . Almonds and postprandial glycemia—a dose–response study. Metabolism 2007; 56: 400–404.
Ma Y, Njike VY, Millet J, Dutta S, Doughty K, Treu JA et al. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabet Care 2010; 33: 227–232.
West SG, Krick AL, Klein LC, Zhao G, Wojtowicz TF, McGuiness M et al. Effects of diets high in walnuts and flax oil on hemodynamic responses to stress and vascular endothelial function. J Am Coll Nutr 2010; 29: 595–603.
Casas-Agustench P, Lopez-Uriarte P, Ros E, Bullo M, Salas-Salvado J . Nuts, hypertension and endothelial function. Nutr Metab Cardiovasc Dis 2011; 21 (suppl 1), 21s–33s.
Kendall CW, Josse AR, Esfahani A, Jenkins DJ . The impact of pistachio intake alone or in combination with high-carbohydrate foods on post-prandial glycemia. Eur J Clin Nutr 2011; 65: 696–702.
West SG, Gebauer SK, Kay CD, Bagshaw DM, Savastano DM, Diefenbach C et al. Diets containing pistachios reduce systolic blood pressure and peripheral vascular responses to stress in adults with dyslipidemia. Hypertension 2012; 60: 58–63.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III). JAMA 2001; 285: 2486–2497.
Green SM, Delargy HJ, Joanes D, Blundell JE . A satiety quotient: a formulation to assess the satiating effect of food. Appetite 1997; 29: 291–304.
McCrea C, Skulas-Ray AC, Chow M, West SG . Test–retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vasc Med 2012; 17: 29–36.
Jackson KG, Wolstencroft EJ, Bateman PA, Yaqoob P, Williams CM . Acute effects of meal fatty acids on postprandial NEFA, glucose and apo E response: implications for insulin sensitivity and lipoprotein regulation? Br J Nutr 2005; 93: 693–700.
Gannon MC, Nuttall JA, Nuttall FQ . Oral arginine does not stimulate an increase in insulin concentration but delays glucose disposal. Am J Clin Nutr 2002; 76: 1016–1022.
Fernstrom JD, Cameron JL, Fernstrom MH, McConaha C, Weltzin TE, Kaye WH . Short-term neuroendocrine effects of a large oral dose of monosodium glutamate in fasting male subjects. J Clin Endocrinol Metab 1996; 81: 184–191.
Nuttall FQ, Gannon MC, Jordan K . The metabolic response to ingestion of proline with and without glucose. Metabolism 2004; 53: 241–246.
Tulipano G, Sibilia V, Caroli AM, Cocchi D . Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides 2011; 32: 835–838.
Ryan AT, Feinle-Bisset C, Kallas A, Wishart JM, Clifton PM, Horowitz M et al. Intraduodenal protein modulates antropyloroduodenal motility, hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr 2012; 96: 474–482.
Baggio LL, Drucker DJ . Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132: 2131–2157.
Drab SR . Incretin-based therapy for type-2 diabetes mellitus: current status and future prospects. Pharmacotherapy 2010; 30: 609–624.
Mori AM, Considine RV, Mattes RD . Acute and second-meal effects of almond form in impaired glucose tolerant adults: a randomized crossover trial. Nutr Metab (Lond) 2011; 8: 6.
Verdich C, Flint A, Gutzwiller E, Näslund E, Beglinger C, Hellström PM et al. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab 2001; 86: 4382–4389.
Vogel RA, Corretti MC, Plotnick GD . Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol 1997; 79: 350–354.
Cortés B, Núñez I, Cofán M, Gilabert R, Pérez-Heras A, Casals E et al. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J Am Coll Cardiol 2007; 17: 1666–1671.
Vogel RA, Corretti MC, Plotnick GD . The postprandial effect of components of the Mediterranean diet on endothelial function. J Am Coll Cardiol 2000; 36: 1455–1460.
Acknowledgements
This work was supported by the American Pistachio Growers, Fresno, CA, USA. DJAJ is funded by the Federal Government of Canada as a Canada Research Chair in Nutrition and Metabolism.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
CWCK has received research grants, travel funding, consultant fees, honoraria or has served on the scientific advisory board for Abbott, Advanced Food Materials Network, Almond Board of California, American Peanut Council, American Pistachio Growers, Barilla, California Strawberry Commission, Canadian Institutes of Health Research, Canola Council of Canada, Danone, General Mills, Hain Celestial, International Tree Nut Council, Kellogg, Loblaw Brands Ltd, Oldways, Orafti, Paramount Farms, Pulse Canada, Saskatchewan Pulse Growers, Solae and Unilever. ALJ is a director of Glycemic Index Laboratories, Toronto, Ontario, Canada. JC and LC are employed by Glycemic Index Laboratories. SGW and KAS have received travel funding and research funding from the American Pistachio Growers. DJAJ reported serving on the Scientific Advisory Board of Unilever, Sanitarium Company, California Strawberry Commission, Loblaw Supermarket, Herbal Life International, Nutritional Fundamental for Health, Pacific Health Laboratories, Metagenics, Bayer Consumer Care, Orafti, Dean Foods, Kellogg’s, Quaker Oats, Procter and Gamble, Coca-Cola, NuVal Griffin Hospital, Abbott, Pulse Canada, Saskatchewan Pulse Growers and Canola Council of Canada; receiving honoraria for scientific advice from the Almond Board of California, International Tree Nut Council Nutrition Research and Education Foundation, Barilla, Unilever Canada, Solae, Oldways, Kellogg’s, Quaker Oats, Procter and Gamble, Coca-Cola, NuVal Griffin Hospital, Abbott, Canola Council of Canada, Dean Foods, California Strawberry Commission, Haine Celestial and Alpro Foundation; being on the speakers panel for the Almond Board of California; receiving research grants from Loblaw Brands Ltd, Unilever, Barilla, Almond Board of California, Solae, Haine Celestial, Sanitarium Company, Orafti, International Tree Nut Council and Peanut Institute; and receiving travel support to meetings from the Almond Board of California, Unilever, Alpro Foundation and International Tree Nut Council. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kendall, C., West, S., Augustin, L. et al. Acute effects of pistachio consumption on glucose and insulin, satiety hormones and endothelial function in the metabolic syndrome. Eur J Clin Nutr 68, 370–375 (2014). https://doi.org/10.1038/ejcn.2013.275
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2013.275
Keywords
This article is cited by
-
Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence
Nature Reviews Nephrology (2021)
-
Brazil nut consumption promotes satiety without increasing blood glucose and insulin responses in healthy adults
Nutrire (2020)
-
Nutritional modulation of endogenous glucagon-like peptide-1 secretion: a review
Nutrition & Metabolism (2016)
-
Nut-enriched bread is an effective and acceptable vehicle to improve regular nut consumption
European Journal of Nutrition (2016)
-
Post-prandial glucose and insulin responses of hummus alone or combined with a carbohydrate food: a dose–response study
Nutrition Journal (2015)