Abstract
Background/Objectives:
Iron is fundamental to many basic biological functions, and animal studies suggest that iron deficiency early in life can have a lasting impact on the developing brain.
Subjects/Methods:
We used a population-based cohort of mothers and their children to assess the effect of iron status among pregnant women on the cognitive ability of their offspring. But to avoid the inherent confounding that occurs within observational epidemiology studies we examined the association of maternal genotype at single-nucleotide polymorphisms in the genes HFE (rs1799945) and (rs1800562), TF (rs3811647) and TMPRSS6 (rs1800562), which are related to iron, haemoglobin or transferrin levels, on their child’s cognitive test scores at age 8.
Results:
We found strong associations between HFE and TMPRSS6 genotypes and mother’s haemoglobin levels early in pregnancy (P-values are all ⩽4.1 × 10−5) and a genetic score comprised of alleles at these loci was even more strongly associated with haemoglobin levels (P=3.0 × 10−18), suggesting that this was a good instrument to use to look at the effect of prenatal iron levels on offspring cognition. However, mother’s genotype at the above loci was not associated with offspring IQ at age 8.
Conclusions:
We therefore concluded that there is no evidence of an effect of exposure to low levels of iron (within the normal range) in pregnancy on offspring cognition at age 8. However, pregnant women in the UK with low haemoglobin levels are prescribed iron supplements and so we were unable to look at the effect of iron deficiency in our study.
Similar content being viewed by others
Introduction
Iron is fundamental to many basic biological functions including oxygen transport, production of adenosine triphosphate (ATP), DNA synthesis, mitochondrial function and protection of cells from oxidative damage.1 However, iron deficiency is the most common nutritional deficiency worldwide, with around 1.6 billion people thought to be affected. This is particularly the case in developing countries where 25% of pregnant women are reported to be iron deficient.2
Animal studies suggest that iron deficiency early in life can have a lasting impact on the developing brain.3 In humans, the brain is likely to be most vulnerable to nutrient deficiencies during the brain growth spurt that occurs in the period between the last trimester of fetal life and the first 2 years of childhood.3
A recent meta-analysis of five randomized controlled trials of early life iron supplementation on motor and mental development in children under 3 years of age found evidence of a beneficial effect on motor development and very weak evidence of a beneficial effect on mental development.4 The same review found only one randomized controlled trial that specifically addressed the effect of prenatal iron supplementation on child’s IQ (at age 4). This study was carried out in Australia, and showed no differences in IQ between the two groups. There was, however, a very high drop-out rate (30%), so the sample used for the final analysis may not be representative of those randomized.5 Further intervention studies of iron supplementation among pregnant women would need robust evidence to justify their conduct, as around half of all pregnant women require iron supplements to prevent anaemia when pregnant. Although observational studies are feasible, they are limited in their ability to examine whether maternal iron status in pregnancy affects offspring neurological development and cognitive function, because they are likely to be confounded by socio-economic background, and related lifestyle factors, which are strongly associated with both nutritional status6 and childhood cognitive function.7
Associations between genetic polymorphisms, which are associated with iron levels, and cognition are less likely to be subject to the problem of confounding by lifestyle factors, which occurs in observational studies,8 and studies of this type are not subject to the ethical issues that arise in randomized controlled trials.9 We used genetic variants known to be associated with iron levels to determine whether maternal iron status during pregnancy was causally associated with child’s IQ score at age 8 in a large population-based study.
Materials and methods
Study population
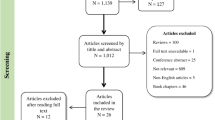
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a population-based prospective study investigating factors that affect the health and development of children and their parents. The study methods are described in detail elsewhere (http://www.alspac.bris.ac.uk10, 11, 12). In brief, pregnant women living in Bristol, UK who had an expected date of delivery between April 1991 and December 1992 were eligible. A total of 14 541 pregnant women enrolled in the study. A detailed outline of the exclusion criteria for this analysis and numbers with missing data is given in Figure 1. Extensive data have been collected from the mothers from pregnancy onwards by questionnaire, abstraction from medical notes, record linkage and by attendance at research clinics. Ethical approval for the study was obtained from the ALSPAC law and ethics committee and the local research ethics committees.
Ethnicity
We excluded all non-white women from this analysis to avoid population stratification (see Figure 1 for exclusions). Ethnicity was available from self-report or had been imputed from five genetic ancestry-informative markers with established population-specific allelic distributions: rs713598 and rs1726866 in TAS2R38,13 rs4988235 in MCM6,14 rs41310927 in ASPM15 and rs930557 in MCPH1.15
Measurement of cognition
Cognitive testing of children was carried-out during a clinical visit when the children were aged 8 using a shortened version of the Wechsler Intelligence Scale for Children (WISC-III), described in detail elsewhere.16 An overall age-adjusted cognitive score was derived from this assessment for each child who completed the test.17 This test has been shown to be associated with mother’s educational level, parity, socio-economic status and several other socio-economic and lifestyle factors in a previous analysis, thus we are confident of the validity of this test (supplementary tables in Bonilla et al.18).
Measurement of mothers educational level
Mother’s educational level was obtained by questionnaires administered to the mother during pregnancy, in which women were asked to report their highest educational attainment from five-ordered categories. For the purposes of adjustment in the models of mother’s genotype and child’s IQ, the five categories were used. However, when mother’s education was used as the outcome, these categories were collapsed as follows: ordinary level (O-level) or equivalent vs higher or lower. The O-level was an exam-based qualification for students aged 14–16 years (16 is the legal minimum school leaving age in the UK), which was replaced by the General Certificate of Secondary Education (GCSE) in 1988 in the UK. Further details are available at http://www.direct.gov.uk/en/EducationAndLearning/QualificationsExplained/DG_10039024.
Iron supplementation
ALSPAC mothers were asked at 18 and 32 weeks of pregnancy whether they had taken iron supplements during this pregnancy, they were also asked to list any medications they were taking as a text answer. If mothers reported taking iron supplements and/or taking medications or supplements containing iron, they were classed as taking iron supplements. We constructed three groups as follows: (1) not taking iron supplements, women who did not report taking supplements or medication containing iron at 18 or 32 weeks of pregnancy; (2) taking iron supplements at 18 weeks, women who reported taking supplements or medication containing iron at 18 weeks of pregnancy, irrespective of whether they were taking supplements at 32 weeks or not; (3) taking iron supplements at 32 weeks, women who reported taking supplements or medication containing iron at 32 weeks but not at 18 weeks of pregnancy.
Measurement of confounders
Data on selected characteristics from ALSPAC mothers were used to conduct an assessment of the potential for confounding of the genotype–outcome association. These were collected either from hospital records or from the questionnaires completed by the mother during pregnancy. Mother’s social class was based on occupation and determined according to the 1991 British Office of Population Statistics classification. Marital status, housing tenure, parity, inter-pregnancy interval, breastfeeding, infection during pregnancy, ever smoked, alcohol consumption before supplements during pregnancy, calcium supplements during pregnancy, folate supplements during pregnancy, other supplements and sex of the child were all tested as potential confounders.
Laboratory methods
DNA was extracted using the salting out method. This was part of a larger study in which 58 single-nucleotide polymorphisms (SNPs) related to nutrient intake or levels were genotyped to look at the effect of early life nutrition on child’s IQ score. In addition, a genome-wide association study was later carried out in this cohort. Four SNPs previously shown to cause haemochromatosis19 or to be strongly associated (for all P<4 × 10−6) with serum iron, serum transferrin or haemoglobin20 were genotyped, these were HFE rs1800562, rs1799945; TF rs3811647; and TMPRSS6 rs4820268. A summary of what is known about these SNPs is given in Text Box 1. Genotyping was undertaken by KBioscience Ltd (www.kbioscience.co.uk), who use their own form of competitive allele-specific PCR system (KASPar) and Taqman for SNP analysis. KBiosciences have their own quality control checks. They state that to deem an assay successful it must possess three distinct clusters: the water controls must be negative, the number of genotypes callable must be >90% and the minor allele frequency should be >2%. In addition, we added blind duplicates to the samples, we checked that there were in fact three true clusters for each genotype, and where there was evidence that an SNP was not in Hardy–Weinberg equilibrium, we looked at the clusters by eye to verify whether there was any evidence of miscalling of genotypes or whether any of the unresolved genotypes could be called.
Diplotypes and genotype score
As haemochromatosis can arise from having a rare homozygous genotype at rs1799945 or rs1800562 in the HFE gene or by being a composite heterozygote with one rare allele at each site, we combined genotypes at these two loci as follows: two low-iron alleles (any two haemochromatosis alleles); three low-iron alleles (heterozygotes at just one locus, with the common homozygous genotype at the other locus); four low-iron alleles (double homozygotes for common alleles).
Genotype score comprised alleles at three loci, the two above in HFE and rs4820268 inTMPRSS6, with risk alleles defined as those associated with lower haemoglobin levels, that is, allele C at rs1799945, G at rs1800562 and G at rs4820268. Individuals were categorised according to whether they had 2 or 3, 4, 5 or 6 risk alleles. No individual had 0 or 1 low iron allele.
Measurement of haemaglobin in ALSPAC
Mother’s haemoglobin levels were measured routinely at various time points during pregnancy by healthcare workers and were extracted from obstetric notes. The levels used to assess associations with outcome and genotype were the first measurement taken during pregnancy, with most (87%) taken before 18 weeks of pregnancy, and the last measurement taken during pregnancy (for this we specified that the measurement should have been done after 28 weeks of pregnancy).
Statistical analysis
Ordered logistic regression analysis was carried out in Stata (Stata Corporation, College Station, TX, USA) to test for associations of mother’s educational level (<O-level, O-level, >O-level) with genotype without adjustment and with adjustment for population stratification. Associations between supplement use (not taking supplements, taking supplements at 18 weeks and taking supplements at 32 weeks only) and genotype, and categorical confounders and genotype were tested using χ2-tests. Linear regression models were used to examine the association of maternal genotype (exposure) with WISC score or haemoglobin as outcome, and to test the association between mother’s haemoglobin level and child’s WISC score. Per allele odds ratios are reported for individual genotypes/genotype scores.
We carried out crude analyses of mother’s genotype and child’s WISC score, we also carried out an analysis adjusting for: child’s genotype (to assess whether mother’s genotype was exerting an independent effect on outcome rather than affecting outcome by influencing child’s genotype); mother’s educational level (there were two reasons for adjusting for this—first, in a sensitivity analysis where we adjusted for all potential confounders, only mother’s educational level exerted an independent effect on outcome and second, we wanted to exclude the possibility that mother’s genotype was influencing outcome by affecting her own educational attainment); we also adjusted for population stratification using the top 10 principal components (PCs), from genome wide association data available in ALSPAC, that reflect the population’s genetic structure estimated according to Price et al.21 Assumptions of Hardy–Weinberg equilibrium were formally tested using a likelihood ratio test and the asymptotic P-value is reported. All the above analysis was carried-out using Stata 11.0.
Results
The TMPRSS6 rs4820268 polymorphism showed evidence of Hardy–Weinberg disequilibrium in the mothers (P=0.001) but not in the children in ALSPAC (Table 1), this was due to a slight excess in heterozygotes compared with the number expected. We studied the raw data, but could find no evidence of miscalling or that homozygotes were over-represented among the women for whom genotype was not resolved. All other genotypes were in accordance with Hardy–Weinberg equilibrium in ALSPAC. Therefore no genotype was excluded on the basis of Hardy–Weinberg equilibrium.
Mother’s haemoglobin levels by genotype in early pregnancy
We found a strong association between HFE genotypes and haemoglobin levels measured early in pregnancy (Table 2) and similar effects with haemoglobin measured late in pregnancy (results not shown), with rare homozygotes having higher levels compared with wild-type homozygotes and levels among heterozygotes being intermediate between the two. TMPRSS6 rs4820268 was also strongly associated with haemoglobin levels at both time points with the rare homozygote GG having the lowest levels. The TF SNP investigated in this study did not show evidence of being associated with haemoglobin levels, for this reason this SNP was not included in the genotype score. Both the HFE diplotype and the genotype score based on both HFE genotypes and TMPRSS6 rs4820268 explained more of the variation in haemoglobin levels than individual SNPs alone, with the genotype score showing very strong evidence of a dose–response relationship with haemoglobin levels, but still only explaining 1.02% of the variation in early pregnancy haemoglobin levels.
Genotypes and iron supplementation during pregnancy among ALSPAC mothers
Table 3 shows associations between taking iron supplements during pregnancy and genotype. Both the HFE loci were associated with taking supplements during pregnancy such that women with rare homozygote genotypes were less likely to take supplements, with some evidence of a dose response (albeit weak evidence). Women carrying the TMPRSS6 rs4820268 G allele were more likely to take supplements during pregnancy, and there seemed to be a per allele effect for this genotype. The TF rs3811647 SNP showed no clear pattern of association with taking supplements. Although there was weak evidence that women with the heterozygous genotype were less likely to take supplements. Furthermore, HFE diplotypes and a genotypic score containing both HFE SNPs and TMPRSS6 rs4820268 were stronger predictors of supplement use than individual genotypes.
Genotypes and potential confounders
We looked at the association between genotype/diplotype/genotype score in mothers and 19 potential confounding factors. We found evidence for a small number of associations at P=0.05 level, which are as follows: HFE rs1800562 and alcohol intake before pregnancy (P=0.02), HFE rs1800562 and social class (P=0.02), HFE rs1800562 and taking folate supplementation (P=0.001), TMPRSS6 rs4820268 and parity (P=0.01), These four associations are 5.3% of the total number of genotype–potential confounder associations tested (that is, the expected number (5%) due to chance). Associations between confounders and diplotypes/genotype score largely reflected associations with individual genotypes and were as follows: HFE diplotypes and social class (P=0.001); HFE diplotypes and folate supplementation (P=0.001); genotype score and infection during pregnancy (P=0.003); and genotype score and folate supplementation (P=0.001).
Genotype and mothers’ educational level
We found associations between mothers’ educational level and HFE rs1799945 genotypes, such that mothers with the rare allele (associated with higher iron levels) were more likely to have an educational level that was greater than O-level (Table 4). We also found an association between the TF rs3811647 genotype and mother’s educational level, such that the A allele (associated with higher transferrin levels) was associated with a higher educational attainment. Adjustment for population stratification did not change these results.
Mother’s haemoglobin level in pregnancy and child’s IQ
Mother’s haemoglobin levels early in pregnancy among mothers on whom we had offspring IQ data were on average (mean) 12.3 g/dl, with a range of 7.2–16.2, and only 7.8% of mothers had levels considered to be low (<11 g/dl). If we take the last haemoglobin measurement that was available during pregnancy, the mean haemoglobin level during pregnancy among mothers who had children with WISC data was 11.4 g/dl, range 7.3–15.7, with 30% of mothers having haemoglobin levels of <11 g/dl. Mothers’ haemoglobin level (g/dl) early in pregnancy (adjusted by gestation in days when measured) was not found to be associated with child’s WISC score at age 8 (mean difference in WISC score per g/dl=0.01 (95% confidence interval (CI)=−0.44 to 0.46), P=0.96) even after the adjustment for iron supplement intake during pregnancy (mean difference=0.11 (95% CI=−0.37 to 0.60) P=0.65). There was weak evidence that haemoglobin levels measured after 28 weeks of pregnancy (adjusted by gestation in days when measured) were associated with the child’s IQ score (mean difference in WISC score per g/dl=0.41 (95% CI=−0.07 to 0.46), P=0.09), but this effect disappeared after adjustment for potential confounders g/dl=0.01 (95% CI=−0.53 to 0.51), P=0.98).
Mother’s genotype and child’s IQ score
We found no evidence of associations between mother’s genotype and child’s WISC score at age 8 (Table 5) in our crude analysis and in an analysis adjusted by iron supplementation, child’s genotype, mother’s education level and population stratification.
Discussion
We found strong evidence of associations between two SNPs in HFE, which have been previously shown to cause iron overload, and haemoglobin levels during early pregnancy in this large population based study of women. We also found strong associations between rs4820268 in TMPRSS6 and haemoglobin levels. In addition, an iron genotype score that comprised of the above three SNPs was associated (P=1.9 × 10−18) with a 0.3 s.d. difference in haemoglobin levels. Therefore, we concluded that we had a good genetic instrument with which to determine whether iron levels during pregnancy were associated with offspring cognition. We did not find any evidence for an association between TF rs3811647 and haemoglobin levels, although the A allele at this site has previously been reported to be associated with serum transferrin and reduced transferrin saturation,20 it has not previously been demonstrated to be associated with haemoglobin to our knowledge.
The association between mother’s genotype and haemoglobin was strong at the beginning of pregnancy, and we expected this association to be less evident later on in pregnancy, as women in the UK with low iron levels during pregnancy are advised by a medical practitioner to take supplements. Those genotypes associated with low haemoglobin levels are also associated with an increased likelihood of receiving iron supplements, and supplementation will remove or at least diminish an association between genotype and iron levels. However, we tested by comparing genotypes with haemoglobin later in pregnancy and found that the associations were as strong.
We found no evidence that low iron levels in pregnancy have a detrimental effect on the developing fetus’ brain, either in the observational analysis (using haemoglobin measured in early and late pregnancy) or using genotypes in the Mendelian randomization approach. Our study had sufficient power to detect a 0.80-unit change in IQ score per g/dl increase in haeomglobin with a P-value <0.05 (assuming 80% power) in the observational analysis. As a 1-g/dl change in haemoglobin levels is quite large, this is likely to be at the lower end of what is clinically relevant, thus we were satisfied that we had sufficient power to detect an effect in the observational analysis. The power to detect an effect in the Mendelian randomization analysis is much lower; however, as we did not see an effect of haemoglobin in the observational analysis, we are not concerned that we missed an important effect of prenatal exposure to iron on childhood IQ due to low power in the study. There are several limitations to this analysis, which are described below. The first is that while nutrient intake and therefore haemoglobin levels of the mother during pregnancy are expected to be strongly confounded by socio-economic factors, the expectation is that genotype will not be associated with these factors.8 In this study, we found some evidence that rare alleles in HFE rs1800562 and TF rs3811647 were associated with an increased educational attainment among ALSPAC mothers. These variants are both likely to have an effect on overall iron availability. The rare variant in HFE rs1800562 causes iron overload and is associated with an increase in haemoglobin levels,19, 22 whereas the rare allele in TF rs3811647 is associated with higher transferrin levels20 and lower transferrin saturation.20 But more importantly for this study, as maternal educational level is one of the strongest predictors of childhood cognition, this violates the assumption of no confounding by genotype. However, adjustment of our mothers’ genotype–childs’ IQ analysis by mother’s educational level did not change our conclusions. The frequency of the alleles used in this analysis differs widely by population, so there is also the potential for any association to be confounded by population structure. However, we adjusted our analysis by the top 10 principal components that reflect the population structure from GWAS data carried out on this population and found that this adjustment did not affect our conclusions.
The HFE variant rs1800562 that was associated with educational status was also associated with social class, which may be a chance finding or may be a consequence of the association with educational status, as the two are closely linked. Similarly, the association between genotypes at this loci and taking folic acid supplements could be due to chance or could be because many women taking iron supplements may take iron with folic acid.
A further limitation of this study is that the number of women with HFE rare homozygous genotypes were small and our study therefore does not have any power to detect effects with these genotypes. Replication in a larger study would be desirable to confirm our findings; however, we are not aware of any large studies with DNA from mothers and IQ from children that could readily do this analysis.
Finally, as this is a relatively well-nourished population who were closely monitored during pregnancy, and given supplements if their iron levels were found to be low, it is possible that the haemoglobin levels in this study simply did not fall low enough to be detrimental to the fetus. By the end of pregnancy 30% of the mothers had levels that are generally considered to be low. However, it is not known whether these are sufficiently low as to affect cognitive development in their offspring, indeed it is likely that levels in the mothers are low, because iron is preferentially diverted to the fetus to negate any deleterious effects.
In conclusion, our results do not support the hypothesis that exposure to low levels of iron early in fetal life adversely affect brain development and therefore IQ in childhood. However, our findings should be interpreted with caution owing to the fact that pregnant women with low haemoglobin levels in the UK receive iron supplements fairly early on in pregnancy; thus, we are unable to study the effects of low iron in the second and third trimester of pregnancy in this cohort.
References
Atamna H, Walter PB, Ames BN . The role of heme and iron-sulfur clusters in mitochondrial biogenesis, maintenance, and decay with age. Arch Biochem Biophys 2002; 397: 345–353.
WHO 2008. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia Benoist Bruno de, McLean Erin, Egli Ines, Cogswell Mary (eds), WHO Press: Geneva, Switzerland, 2008.
Kolb B, Whishaw IQ . An introduction to brain and behaviour . 1st edn. Worth Publishers: New York, NY, USA, 2001.
Szajewska H, Ruszczynski M, Chmielewska A . Effects of iron supplementation in nonanemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: a systematic review of randomized controlled trials. Am J Clin Nutr 2010; 91: 1684–1690.
Zhou SJ, Gibson RA, Crowther CA, Baghurst P, Makrides M . Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 y of age: long-term follow-up of a randomized controlled trial. Am J Clin Nutr 2006; 83: 1112–1117.
De Irala-Estevez J, Groth M, Johansson L, Oltersdorf U, Prättälä R, Martinez-Gonzalez MA . A systematic review of socio-economic differences in food habits in Europe: consumption of fruit and vegetables. Eur J Clin Nutr 2000; 54: 706–714.
Lawlor DA, Clark H, Davey Smith G, Leon DA . Intrauterine growth and intelligence within sibling-pairs: findings from the Aberdeen Children of the 1950 s cohort. Pediatrics 2006; 117: e894–e902.
Davey Smith G, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S . Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med 2007; 4: e352.
Davey Smith G, Ebrahim S . 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003; 32: 1–22.
Golding J, ALSPAC. Children of the nineties: a resource for assessing the magnitude of long-term effects of prenatal, perinatal and subsequent events. Obstetrics 1996; 8: 89–92.
Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G et al. Cohort Profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 2013; 42: 97–110.
Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J et al. Cohort Profile: The “Children of the 90 s”—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2012; 42: 111–127.
Timpson NJ, Christensen M, Lawlor DA, Gaunt TR, Day IN, Ebrahim S et al. TAS2R38 (phenylthiocarbamide) haplotypes, coronary heart disease traits, and eating behavior in the British Women’s Heart and Health Study. Am J Clin Nutr 2005; 81: 1005–1011.
Swallow DM . Genetics of lactase persistence and lactose intolerance. Annu Rev Genet 2003; 37: 197–219.
Timpson N, Heron J, Smith GD, Enard W . Comment on papers by Evans et al. and Mekel-Bobrov et al. on evidence for positive selection of MCPH1 and ASPM. Science 2007; 317: 1036a.
Joinson C, Heron J, Butler R, Von Gontard A, Butler U, Emond A et al. A United Kingdom population-based study of intellectual capacities in children with and without soiling, daytime wetting, and bed-wetting. Pediatrics 2007; 120: e308–e316.
Wechsler D . Manual for the Wechsler Intelligence Scale for Children 3rd edn Psychological Corporation: San Antonio, TX, 1991.
Bonilla C, Lawlor DA, Taylor AE, Gunnell DJ, Ben-Shlomo Y, Ness AR et al. Vitamin B-12 status during pregnancy and child's IQ at age 8: a Mendelian randomization study in the Avon longitudinal study of parents and children. PLoS One 2012; 7: e51084.
Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet 1996; 13: 399–408.
Benyamin B, McRae AF, Zhu G, Gordon S, Henders AK, Palotie A et al. Variants in TF and HFE explain approximately 40% of genetic variation in serum-transferrin levels. Am J Hum Genet 2009; 84: 60–65.
Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D . Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006; 38: 904–909.
Chambers JC, Zhang W, Li Y, Sehmi J, Wass MN, Zabaneh D et al. Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat Genet 2009; 41: 1170–1172.
Finberg KE, Heeney MM, Campagna DR, Aydinok Y, Pearson HA, Hartman KR et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet 2008; 40: 569–571.
Delanghe J, Verstraelen H, Pynaert I, Debels L, Taes Y, Verhasselt B et al. Human transferrin G277S mutation and iron deficiency in pregnancy. Br J Haematol. 2006; 132: 249–250.
Acknowledgements
We are grateful to all the women and children who took part in this study, the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. This work is supported by a Wellcome Trust grant (grant ref: WT084668MA), which funds CB. DA Lawlor and G Davey Smith work in an MRC Centre that is supported by the UK Medical Research Council (G0600705) and the University of Bristol. DG is an NIHR Senior Investigator. The UK Medical Research Council (grant ref: 74882), the Wellcome Trust (grant ref: 076467) and the University of Bristol provide core support for ALSPAC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
SJL and GDS designed the research project. SJL decided on the SNPs to analyse and ordered the genotyping, CB and MJB analysed the data. DAL, DG, sYB-S and AN advised on the data analysis and interpretation of the data, SL wrote the paper and had primary responsibility for final content. All authors commented on earlier drafts of the manuscript and have read and approved the final manuscript.
Rights and permissions
This work is licensed under a Creative Commons Attribution 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/
About this article
Cite this article
Lewis, S., Bonilla, C., Brion, MJ. et al. Maternal iron levels early in pregnancy are not associated with offspring IQ score at age 8, findings from a Mendelian randomization study. Eur J Clin Nutr 68, 496–502 (2014). https://doi.org/10.1038/ejcn.2013.265
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2013.265
Keywords
This article is cited by
-
Methodological approaches, challenges, and opportunities in the application of Mendelian randomisation to lifecourse epidemiology: A systematic literature review
European Journal of Epidemiology (2023)
-
A systematic literature review of the relation between iron status/anemia in pregnancy and offspring neurodevelopment
European Journal of Clinical Nutrition (2019)
-
Iron deficiency and iron treatment in the fetal developing brain – a pilot study introducing an experimental rat model
Reproductive Health (2018)
-
The effect of universal maternal antenatal iron supplementation on neurodevelopment in offspring: a systematic review and meta-analysis
BMC Pediatrics (2018)
-
Association between maternal nutritional status in pregnancy and offspring cognitive function during childhood and adolescence; a systematic review
BMC Pregnancy and Childbirth (2016)