Abstract
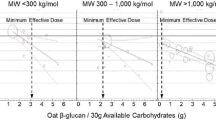
Oat and barley foods have been shown to reduce human glycaemic response, compared to similar wheat foods or a glucose control. The strength of the evidence supporting the hypothesis that the soluble fibre, mixed linkage β-glucan, reduces glycaemic response was evaluated. A search of the literature was conducted to find clinical trials with acute glycaemic response as an end point using oat or barley products. Of the 76 human studies identified, 34 met the inclusion and exclusion criteria. Dose response and ratio of β-glucan to available carbohydrate as predictors of glycaemic response were assessed. Meals provided 0.3–12.1 g oat or barley β-glucan, and reduced glycaemic response by an average of 48±33 mmol·min/l compared to a suitable control. Regression analysis on 119 treatments indicated that change in glycaemic response (expressed as incremental area under the post-prandial blood-glucose curve) was greater for intact grains than for processed foods. For processed foods, glycaemic response was more strongly related to the β-glucan dose alone (r2=0.48, P<0.0001) than to the ratio of β-glucan to the available carbohydrate (r2=0.25, P<0.0001). For processed foods containing 4 g of β-glucan, the linear model predicted a decrease in glycaemic response of 27±3 mmol·min/l, and 76% of treatments significantly reduced glycaemic response. Thus, intact grains as well as a variety of processed oat and barley foods containing at least 4 g of β-glucan and 30–80 g available carbohydrate can significantly reduce post-prandial blood glucose.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jenkins DJA, Wolever TMS, Taylor RH, Ghafari H, Jenkins AL, Barker H et al. Rate of digestion of foods and postprandial glycaemia in normal and diabetic subjects. Brit Med J 1980; 2: 14–17.
Wolever TMS . The Glycaemic Index: A Physiological Classification of Dietary Carbohydrates. CAB International: Oxford, UK, 2006.
Wood PJ . Oat and rye β-glucan: properties and function. Cereal Chem 2010; 87: 315–330.
Ren Y, Ellis PR, Ross-Murphy SB, Wang Q, Wood PJ . Dilute and semi-dilute solution properties of (1–3)(1–4)-β-D-glucan, the endosperm cell wall polysaccharide of oats (Avena sativa L.). Carbohydr Polym 2003; 53: 401–408.
Marciani L, Gowland PA, Spiller RC, Manoj P, Moore RJ, Young P et al. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am J Physiol Gastrointest Liver Physiol 2001; 280: G1227–G1233.
Braaten JT, Wood PJ, Scott FW, Riedel KD, Poste LM, Collins MW . Oat gum, a soluble fiber which lowers glucose and insulin in normal individuals after an oral glucose load: comparison with guar gum. Am J Clin Nutr 1991; 53: 1425–1430.
Panahi S, Ezatagha A, Temelli F, Vasanthan T, Vuksan V . β-Glucan from two sources of oat concentrates affect postprandial glycemia in relation to the level of viscosity. J Am Coll Nutr 2007; 26: 639–644.
Liljeberg H, Granfeldt Y, Bjorck I . Metabolic responses to starch in bread containing intact kernels versus milled flour. Eur J Clin Nutr 1992; 46: 561–575.
Granfeldt Y, Liljeberg H, Drews A, Newman R, Björck I . Glucose and insulin responses to barley products: Influence of food structure and amylose-amylopectin ratio. Am J Clin Nutr 1994; 59: 1075–1082.
Regand A, Chowdhury Z, Tosh SM, Wolever TMS, Wood P . The molecular weight, solubility and viscosity of oat beta-glucan affect human glycemic response by modifying starch digestibility. Food Chem 2011; 129: 297–304.
Wood PJ, Arrigoni E, Miller SS, Amadò R . Fermentability of oat and wheat fractions enriched in β-glucan using human fecal inoculation. Cereal Chem 2002; 79: 445–454.
Queenan KM, Stewart ML, Smith KN, Thomas W, Fulcher RG, Slavin JL . Concentrated oat β-glucan, a fermentable fiber, lowers serum cholesterol in hypercholesterolemic adults in a randomised controlled trial. Nutr J 2007; 6: 6.
Battilana P, Ornstein K, Minehira K, Schwarz JM, Acheson K, Schneiter P et al. Mechanisms of action of β-glucan in postprandial glucose metabolism in healthy men. Eur J Clin Nutr 2001; 55: 327–333.
Nilsson AC, Östman EM, Holst JJ, Björck IME . Including indigestible carbohydrates in the evening meal of healthy subjects improves glucose tolerance, lowers inflammatory markers, and increases satiety after a subsequent standardized breakfast. J Nutr 2008; 138: 732–739.
Nilsson AC, Östman EM, Knudsen KEB, Holst JJ, Björck IME . A cereal-based evening meal rich in indigestible carbohydrates increases plasma butyrate the next morning. J Nutr 2010; 140: 1932–1936.
European Food Safety Authority. Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and ‘digestive function’ (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J 2011; 9: 2207–2228.
Hlebowicz J, Darwiche G, Björgell O, Almér LO . Effect of muesli with 4 g oat β-glucan on postprandial blood glucose, gastric emptying and satiety in healthy subjects: a randomized crossover trial. J Am Coll Nutr 2008; 27: 470–475.
Yokoyama WH, Hudson CA, Knuckles BE, Chiu MCM, Sayre RN, Turnlund JR et al. Effect of barley β-glucan in durum wheat pasta on human glycemic response. Cereal Chem 1997; 74: 293–296.
Tosh SM, Brummer Y, Wolever TMS, Wood PJ . Glycemic response to oat bran muffins treated to vary molecular weight of β-glucan. Cereal Chem 2008; 85: 211–217.
Chillo S, Ranawana DV, Pratt M, Henry CJK . Glycemic response and glycemic index of semolina spaghetti enriched with barley β-glucan. Nutrition 2011; 27: 653–658.
Behall KM, Scholfield DJ, Hallfrisch J, Liljeberg-Elmstahl HGM . Comparison of both resistant starch and β-glucan improves postprandial plasma glucose and insulin in women. Diabetes Care 2006; 29: 976–981.
Cavallero A, Empilli S, Brighenti F, Stanca AM . High (1→3,1→4)-β-glucan barley fractions in bread making and their effects on human glycemic response. J Cereal Sci 2002; 36: 59–66.
De Angelis M, Rizzello CG, Alfonsi G, Arnault P, Cappelle S, Di Cagno R et al. Use of sourdough lactobacilli and oat fibre to decrease the glycaemic index of white wheat bread. Br J Nutr 2007; 98: 1196–1205.
Finocchiaro F, Ferrari B, Gianinetti A, Scazzina F, Pellegrini N, Caramanico R et al. Effects of barley b-glucan-enriched flour fractions on the glycaemic index of bread. Int J Food Sci Nutr 2012; 63: 23–29.
Liljeberg HGM, Granfeldt YE, Björck IME . Products based on a high fiber barley genotype, but not on common barley or oats, lower postprandial glucose and insulin responses in healthy humans. J Nutr 1996; 126: 458–466.
Mäkeläinen H, Anttila H, Sihvonen J, Hietanen RM, Tahvonen R, Salminen E et al. The effect of β-glucan on the glycemic and insulin index. Eur J Clin Nutr 2007; 61: 779–785.
Östman E, Rossi E, Larsson H, Brighenti F, Björck I . Glucose and insulin responses in healthy men to barley bread with different levels of (1→3;1→4)-β-glucans; predictions using fluidity measurements of in vitro enzyme digests. J Cereal Sci 2006; 43: 230–235.
Thondre PS, Wang K, Rosenthal AJ, Henry CJK . Glycaemic response to barley porridge varying in dietary fibre content. Br J Nutr 2012; 107: 719–724.
Alminger M, Eklund-Jonsson C . Whole-grain cereal products based on a high-fibre barley or oat genotype lower post-prandial glucose and insulin responses in healthy humans. Eur J Nutr 2008; 47: 294–300.
Behall KM, Scholfield DJ, Hallfrisch J . Comparison of hormone and glucose responses of overweight women to barley and oats. J Am Coll Nutr 2005; 24: 182–188.
Hallfrisch J, Scholfield DJ, Behall KM . Physiological responses of men and women to barley and oat extracts (Nu-trimX). II. Comparison of glucose and insulin responses. Cereal Chem 2003; 80: 80–83.
Aldughpassi A, Abdel-Aal E-SM . Barley cultivar, kernel composition, and processing affect the glycemic index. J Nutr 2012; 142: 1666–1671.
Grey D, Abdel-Aal E-SM, Seetharaman K, Kakuda Y . Differences in carbohydrate composition and digestion in vitro of selected barley cultivars as influenced by pearling and cooking. Cereal Chem 86: 669–678.
Brummer Y, Duss R, Wolever TMS, Tosh SM . Glycemic response to extruded oat bran cereals processed to vary in molecular weight. Cereal Chem 2012; 89: 255–261.
Casiraghi MC, Garsetti M, Testolin G, Brighenti F . Post-prandial responses to cereal products enriched with barley β-glucan. J Am Coll Nutr 2006; 25: 313–320.
Granfeldt Y, Hagander B, Björck I . Metabolic responses to starch in oat and wheat products. On the importance of food structure, incomplete gelatinization or presence of viscous dietary fibre. Eur J Clin Nutr 1995; 49: 189–199.
Granfeldt Y, Nyberg L, Björck I . Muesli with 4 g oat β-glucans lowers glucose and insulin responses after a bread meal in healthy subjects. Eur J Clin Nutr 2008; 62: 600–607.
Hätönen KA, Similä ME, Virtamo JR, Eriksson JG, Hannila ML, Sinkko HK et al. Methodologic considerations in the measurement of glycemic index: glycemic response to rye bread, oatmeal porridge, and mashed potato. Am J Clin Nutr 2006; 84: 1055–1061.
Hlebowicz J, Wickenberg J, Fahlström R, Björgell O, Almér LO, Darwiche G . Effect of commercial breakfast fibre cereals compared with corn flakes on postprandial blood glucose, gastric emptying and satiety in healthy subjects: a randomized blinded crossover trial. Nutr J 2007; 6: 22.
Holm J, Koellreutter B, Wursch P . Influence of sterilization, drying and oat bran enrichment of pasta on glucose and insulin responses in healthy subjects and on the rate and extent of in vitro starch digestion. Eur J Clin Nutr 1992; 46: 629–640.
Juntunen KS, Niskanen LK, Liukkonen KH, Poutanen KS, Holst JJ, Mykkanen HM . Postprandial glucose, insulin, and incretin responses to grain products in healthy subjects. Am J Clin Nutr 2002; 75: 254–262.
Lan-Pidhainy X, Brummer Y, Tosh SM, Wolever TM, Wood PJ . Reducing beta-glucan solubility in oat bran muffins by freeze-thaw treatment attenuates its hypoglycemic effect. Cereal Chem 2007; 84: 512–517.
Liljeberg H, Bjorck I . Bioavailability of starch in bread products. Postprandial glucose and insulin responses in healthy subjects and in vitro resistant starch content. Eur J Clin Nutr 1994; 48: 151–163.
Nilsson AC, Östman EM, Granfeldt Y, Björck IME . Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am J Clin Nutr 2008; 87: 645–654.
Regand A, Tosh SM, Wolever TMS, Wood PJ . Physicochemical properties of glucan in differently processed oat foods influence glycemic response. J Agric Food Chem 2009; 57: 8831–8838.
Thondre PS, Henry CJK . High-molecular-weight barley β-glucan in chapatis (unleavened Indian flatbread) lowers glycemic index. Nutr Res 2009; 29: 480–486.
Ulmius M, Johansson-Persson A, Krogh M, Olsson P, Önning G . An oat bran meal influences blood insulin levels and related gene sets in peripheral blood mononuclear cells of healthy subjects. Genes Nutr 2011; 6: 429–439.
Wood PJ, Braaten JT, Scott FW, Riedel D, Poste LM . Comparisons of viscous properties of oat and guar gum and the effects of these and oat bran on glycemic index. J Agric Food Chem 1990; 38: 753–757.
Kabir M, Oppert J-M, Vidal H, Bruzzo F, Fiquet C, Würsch P et al. Four-week low-glycemic index breakfast with a modest amount of soluble fibers in type 2 diabetic men. Metabolism 2002; 51: 819–826.
Jenkins AL, Jenkins DJA, Zdravkovic U, Würsch P, Vuksan V . Depression of the glycemic index by high levels of β-glucan fiber in two functional foods tested in type 2 diabetes. Eur J Clin Nutr 2002; 56: 622–628.
Tappy L, Gugolz E, Würsch P . Effects of breakfast cereals containing various amounts of beta-glucan fibers on plasma glucose and insulin responses in NIDDM subjects. Diabetes Care 1996; 19: 831–834.
Tosh SM . Factors affecting bioactivity of cereal β-glucans. In: Salovaara H, Gates F, Tenkanen M, (eds). Dietary Fibre: Conponents and Functions. Wageningen Acedemic. Publishers: Wageningen, The Netherlands, 2007, pp 75–89.
Andersson AAM, Armo E, Grangeon E, Fredriksson H, Andersson R, Aman P . Molecular weight and structure units of (1–3, 1–4)-beta-glucans in dough and bread made from hull-less barley milling fractions. J Cereal Sci 2004; 40: 195–204.
Wolever TMS, Bolognesi C . Source and amount of carbohydrate affect postprandial glucose and insulin in normal subjects. J Nutr 1996; 126: 2798–2806.
Cleary L, Brennan C . The influence of a (1–3)(1–4)-beta-D-glucan rich fraction from barley on the physico-chemical properties and in vitro reducing sugars release of durum wheat pasta. Int J Food Sci Technol 2006; 41: 910–918.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
Tosh, S. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur J Clin Nutr 67, 310–317 (2013). https://doi.org/10.1038/ejcn.2013.25
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2013.25
Keywords
This article is cited by
-
The acute effect of a β-glucan-enriched oat bread on gastric emptying, GLP-1 response, and postprandial glycaemia and insulinemia: a randomised crossover trial in healthy adults
Nutrition & Metabolism (2024)
-
Beneficial glycaemic effects of high-amylose barley bread compared to wheat bread in type 2 diabetes
European Journal of Clinical Nutrition (2023)
-
The importance of molecular weight in determining the minimum dose of oat β-glucan required to reduce the glycaemic response in healthy subjects without diabetes: a systematic review and meta-regression analysis
European Journal of Clinical Nutrition (2023)
-
Greater adherence to the Healthy Nordic Food Index is associated with lower all-cause mortality in a population-based sample from northern Germany
European Journal of Nutrition (2023)
-
Acute effect of Melon Manis Terengganu peel powder on glycemic response, perceived satiety, and food intake: a randomized, placebo-controlled crossover trial in adults at risk of type 2 diabetes
BMC Nutrition (2022)