Abstract
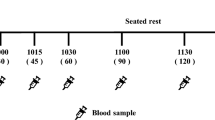
Glycaemic variability challenges the accuracy and use of the glycaemic index (GI). The purpose of the current study was to determine the role of mastication on GI. Using a randomized, controlled, crossover, non-blind design, 15 healthy young subjects returned on 5 separate days for three glucose and two rice test sessions. At the rice sessions, subjects chewed each mouthful either 15 or 30 times. Rice chewed 15 times produced a total glycaemic response (GR; 155 mmol min/l), peak GR (2.4 mmol/l) and GI (68) significantly lower than when chewed for longer (30 times) (184 mmol min/l, 2.8 mmol/l and 88, respectively). The study shows that the GI of rice is affected by the degree of mastication. Chewing 15 times compared with 30 times significantly attenuates the GI, suggesting that mastication may potentially contribute to the glycaemic variability of rice. While future work must establish the extent and limits to which mastication affects glycaemia, it could also explore the potential of using mastication to reduce the glycaemic load of rice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Vega-Lopez S, Ausman LM, Griffith JL, Lichtenstein AH . Interindividual variability and intra-individual reproducibility of glycemic index values for commercial white bread. Diabetes Care 2007; 30: 1412–1417.
Ranawana V, Henry CJK . Liquid and solid carbohydrate foods: comparative effects on glycemic and insulin responses, and satiety. Int J Food Sci Nutr 2011; 62: 71–81.
Ranawana V, Henry CJ, Pratt M . Degree of habitual mastication seems to contribute to interindividual variations in the glycemic response to rice but not to spaghetti. Nutr Res 2010; 30: 382–391.
Ranawana V, Monro JA, Mishra S, Henry CJ . Degree of particle size breakdown during mastication may be a possible cause of interindividual glycemic variability. Nutr Res 2010; 30: 246–254.
Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G et al. Glycaemic index methodology. Nutr Res Rev 2005; 18: 145–171.
ISO 26642-2010. Food products- determination of the glycaemic index (GI) and recommendation for food classification (2010).
Brown WE . Method to investigate differences in chewing behavior in humans: I. Use of electromyography in measuring chewing. J Texture Stud 1994; 25: 1–16.
FAO/WHO. Carbohydrates in human nutrition- Report of a joint FAO/WHO expert consultation. Food and Agriculture Organisation: Rome, 1998.
Wolever TM, Brand-Miller JC, Abernethy J, Astrup A, Atkinson F, Axelsen M et al. Measuring the glycemic index of foods: interlaboratory study. Am J Clin Nutr 2008; 87: 247S–257SS.
Childs N, Burdett A . The US rice export market. Rice Situation and Outlook Yearbook 2000, (RCS-2000): 48–54.
Wolever TMS. The Glycemic Index: a physiological classification of dietary carbohydrate. CABI: Wallingford, 2006.
Ranawana V, Henry CJ . Liquid and solid carbohydrate foods: comparative effects on glycemic and insulin responses, and satiety. Int J Food Sci Nutr 2011; 62: 71–81.
Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009; 301: 2129–2140.
Hu EA, Pan A, Malik V, Sun Q . White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. Br Med J 2012; 344: e1454.
Acknowledgements
This study was supported by the Singapore Institute for Clinical Sciences. We are grateful to Julieana Binte Ibrahim for her help with carrying out the study. VR conducted the research and wrote the paper. ML and JH facilitated the study, contributed to the discussion and reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ranawana, V., Leow, MS. & Henry, C. Mastication effects on the glycaemic index: impact on variability and practical implications. Eur J Clin Nutr 68, 137–139 (2014). https://doi.org/10.1038/ejcn.2013.231
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2013.231
Keywords
This article is cited by
-
Influence of oral processing behaviour and bolus properties of brown rice and chickpeas on in vitro starch digestion and postprandial glycaemic response
European Journal of Nutrition (2022)
-
Increased oral processing and a slower eating rate increase glycaemic, insulin and satiety responses to a mixed meal tolerance test
European Journal of Nutrition (2021)
-
Fruit form influences postprandial glycemic response in elderly and young adults
The Journal of nutrition, health and aging (2017)
-
Mastication and Gut Hormones—Are There Any Associations?
Current Oral Health Reports (2017)